
Close






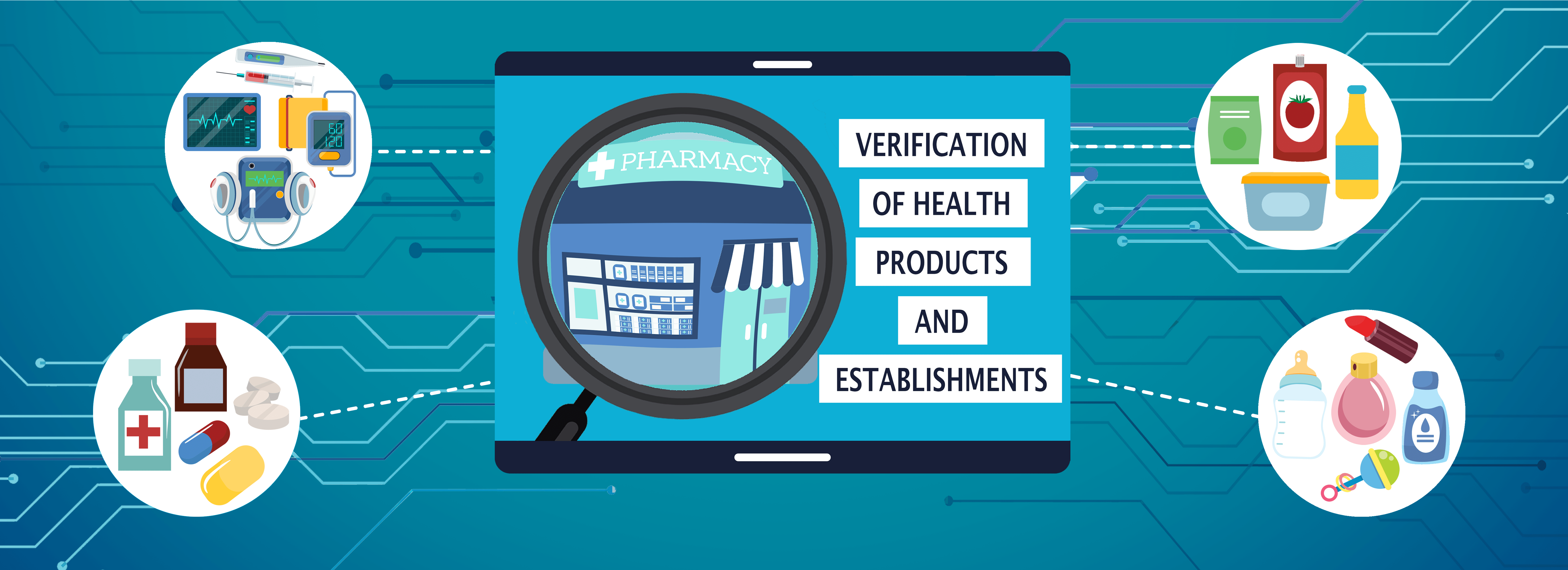

For Products and Establishments registration at FDA
Check the List of Approved FDA
Authorizations
New Online Application System for FDA Authorizations
Apply and Register for License to Operate, Certificate of Product Registration, and other FDA Authorizations

Authorizations for HUHS and Novel Tobacco Products
Applications for Licensing and Registration of Radiation Facilities and Other Related Authorizations

Check the List of Approved FDA
Authorizations

New Online Application System for FDA Authorizations

Apply and Register for License to Operate, Certificate of Product Registration, and other FDA Authorizations

Authorizations for HUHS and Novel Tobacco Products

Applications for Licensing and Registration of Radiation Facilities and Other Related Authorizations



Civic Drive Filinvest Corporate City
(02) 8857-1900
local 1000
(02) 8842-5635
Copyright © 2023. All Rights Reserved.