FDA Advisory NO. 2018-240 Termination of the Voluntary Recall of AV-Set B DT INF-E Blood Tubing System with DVR No. 8574 (Article No. AP16641)
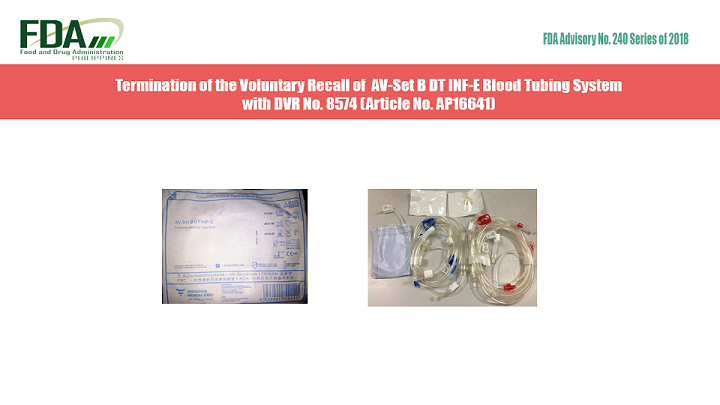
The Food and Drug Administration (FDA) informs the public that Fresenius Medical Care Philippines, Inc., Marketing Authorization Holder (MAH), has reported that it has completed the recall and removal of AV-Set B […]
FDA Advisory No. 2018-236 Public Health Warning Against the Purchase and Use of Unregistered Medical Devices (Weckstat Skin Staple Remover and Weck Visistat 35W Disposable Skin Stapler with 35 Wide Staples)
The Food and Drug Administration (FDA) advises all concerned healthcare professionals and the public against the purchase and use of the following medical device products: FDA post-marketing surveillance activities have […]
Department Memorandum No. 2018-0232 Reiteration of the CSC-DOH Joint Memorandum Circular No. 2010-010 on the “Protection of the bureaucracy against tobacco industry interference”
This is to reiterate the Policy of the Department of Health (DOH) and the Civil Service Commission (CSC) on the Protection of the Bureaucracy Against Tobacco Industry Interference (CSC-DOH JMC […]
FDA Advisory No. 2018-241 Submissions under the Director-General’s Project Backlog

To Facilitate the processing of applications under the “Project Backlog” of the Director-General, and to address the problem on the numerous “incomplete”, “unidentified and “unmatched” applications lodged at the FDA electronic […]
FDA Advisory No. 2018-239 Public Health Warning Against the Purchase and Use of Unregistered Medical Devices included in Johnson & Johnson First Aid To Go

The Food and Drug Administration (FDA) advises the general public and all healthcare professionals against the purchase and use of the unregistered medical devices included in Johnson & Johnson First […]
FDA Advisory No. 2018-238 Public Health Warning Against the Purchase and Use of Unregistered Medical Device Products:

Elastic Bandage 3” Elastic Bandage 4” The Food and Drug Administration (FDA) hereby advises the general public and healthcare professionals against the purchase and use of the following medical device […]
FDA Advisory No. 2018-237 Lifting the Advisory of Indoplas Infrared Ear Thermometer under FDA Advisory No. 2018-120 re: “PUBLIC HEALTH WARNING AGAINST THE PURCHASE AND USE OF THE UNREGISTERED MEDICAL DEVICE PRODUCT (INDOPLAS INFRARED EAR THERMOMETER)”
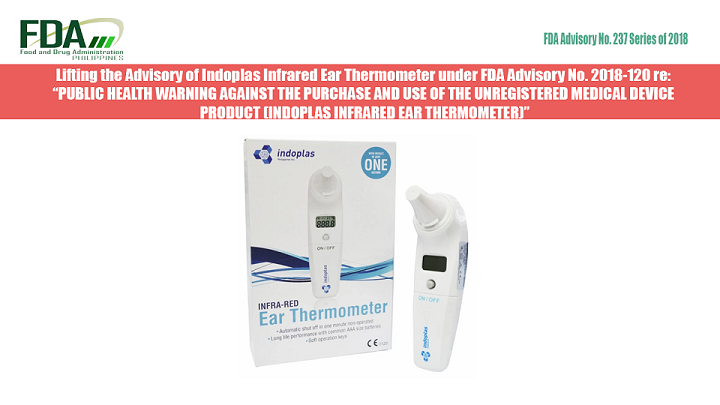
The Food and Drug Administration (FDA) informs the public that the advisory on the medical device product “Indoplas Infrared Ear Thermometer” under FDA Advisory No. 2018-120 dated 02 April 2018 is hereby […]
Follow-Ups, Inquiries and Face-to-face interactions

For effective and efficient public service delivery, the CDRR hereby prescribes the following guidelines for making follow-ups: Status of applications may checked through the “Doctrack Staus” via FDA website https://ww2.fda.gov.ph/index.php/doctrack-status-know-the-status-of-your-application Follow-ups […]
Schedule of CFRR QPIRA Regional Seminars

Schedule of CFRR QPIRA Regional Seminars Please be informed of the schedule of Center for Food Regulation and Research (CFRR) Seminars for 3rd and 4th Quarter of 2018: Course Title […]
FDA Cloud Migration

Please be advised that ICTMD will conduct migration activity (from FDA Server to a Cloud System) starting today 03 August 2018 (6pm) to 5 August 2018 (5pm). Due to this […]



