SUBMISSION OF COURSE REGISTRATION DOCUMENTS FOR WAITLISTED APPLICANTS OF FDA ACADEMY TRAINING SEMINAR

Due to intermittent email error, all waitlisted applicants of FDA Academy Trainings are advised to send their course registration documents to [email protected]. To confirm that your application is a part of […]
SEMINAR VENUE FOR CDRRHR QPIRA SEMINAR (QCDRRHR) ON 2-3 APRIL 2019

Please be informed that the Center for Device Regulation, Radiation Health and Research QPIRA Seminar (QCDRRHR) scheduled on 2-3 April 2019 shall be held at ACACIA HOTEL. VENUE : ACACIA […]
FDA Advisory No. 2019-087-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Hindi Rehistradong Gamot na Ceftriaxone at Sulbactam for Injection USP (Ceftricare™S)
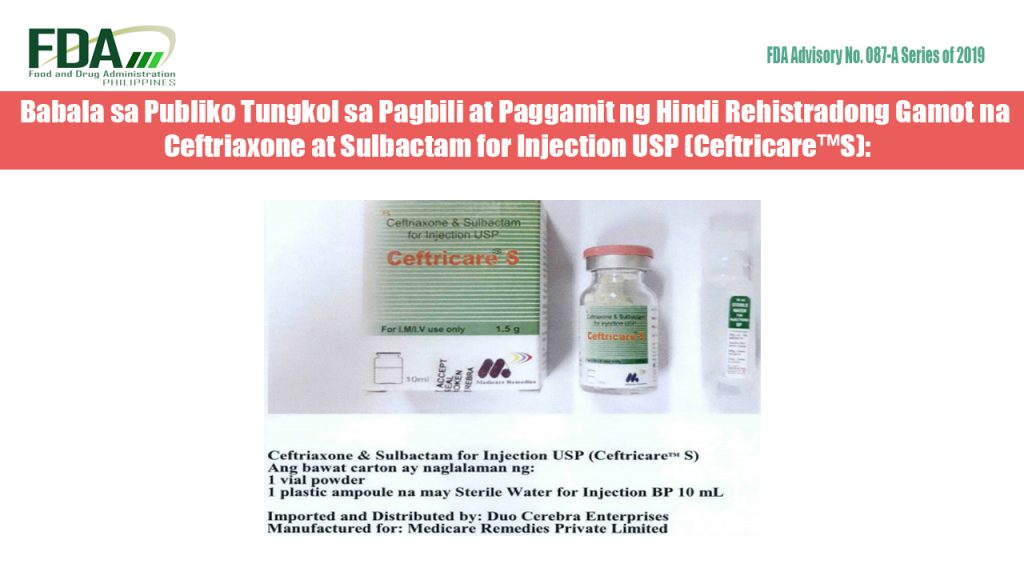
to continue reading, click the link below… FDA Advisory No. 2019-087-A
FDA Advisory No. 2019-087 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product Ceftriaxone & Sulbactam for Injection USP (Ceftricare™S)

to continue reading, click the link below… FDA Advisory No. 2019-087
FDA Advisory No. 2019-086 || Public Health Warning Against the Purchase and Consumption of the Following Unregistered Food Products and Supplements:

to continue reading, click the link below… FDA Advisory No. 2019-086
FDA Advisory No. 2019-085 || Public Health Warning Against the Use of Counterfeit Cosmetic Products (Batch 3)

to continue reading, click the link below… FDA Advisory No. 2019-085
FDA Advisory No. 2019-084 || Dissemination of ASEAN Post-Marketing Alert System(PMAS) Report on Cosmetic Product Containing Banned Ingredient With Reference No. FDA 1008.5 / 327 (8 February 2019)&FDA 1004.03/746(March 2019)

to continue reading, click the link below… FDA Advisory No. 2019-084
FDA Advisory No. 2019-083 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device I-Nex Disposable Sterile Syringe

to continue reading, click the link below… FDA Advisory No. 2019-083
FDA Advisory No. 2019-082 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Supplements:

to continue reading, click the attachment below… FDA Advisory No. 2019-082
FDA Advisory No. 2019-081 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

to continue reading, click the attachment below… FDA Advisory No. 2019-081



