LICENSING SEMINAR FOR ENDS/ENNDS MANUFACTURERS, TRADERS, DISTRIBUTORS, AND RETAILERS

This is to inform all concerned stakeholders that the FDA shall be conducting a licensing seminar for ENDS/ENNDS manufacturers, traders, distributors, and retailers on 4 and 11 September 2019, at […]
VENUE FOR UNIFIED LICENSING SEMINAR (ULS –NCR) ON 5 AND 6 SEPTEMBER 2019

Please be informed that the venue of the Unified Licensing Seminar (ULS – NCR) on 5 and 6 September 2019 will be at the THE BELLEVUE HOTEL MANILA, NORTH BRIDGEWAY, FILINVEST CITY, ALABANG, MUNTINLUPA CITY. All course […]
FDA Advisory No. 2019-256 || Certification of Contraceptive Products in Compliance to the Implementing Rules and Regulations of the Republic Act No. 10354, Also Known as the Responsible Parenthood and Reproductive Health Act of 2012

In compliance with Republic Act No. 10354, also known as “The Responsible Parenthood and Reproductive Health Act of 2012” and its IRR, the Food and Drug Administration (FDA) hereby invites […]
SEMINAR VENUE FOR QPIRA FOR CENTER FOR COSMETICS REGULATION AND RESEARCH (QCCRR-NCR) ON 3-4 SEPTEMBER 2019

Please be informed that the venue of the QPIRA for Center for Cosmetics Regulation and Research (QCCRR-NCR) on 3-4 September 2019 will be at THE BELLEVUE HOTEL, NORTH BRIDGEWAY, FILINVEST CITY, ALABANG, MUNTINLUPA CITY. All course […]
FDA Advisory No. 2019-255 || Voluntary Product Recall of Proximate ILS Curved Intraluminal Stapler
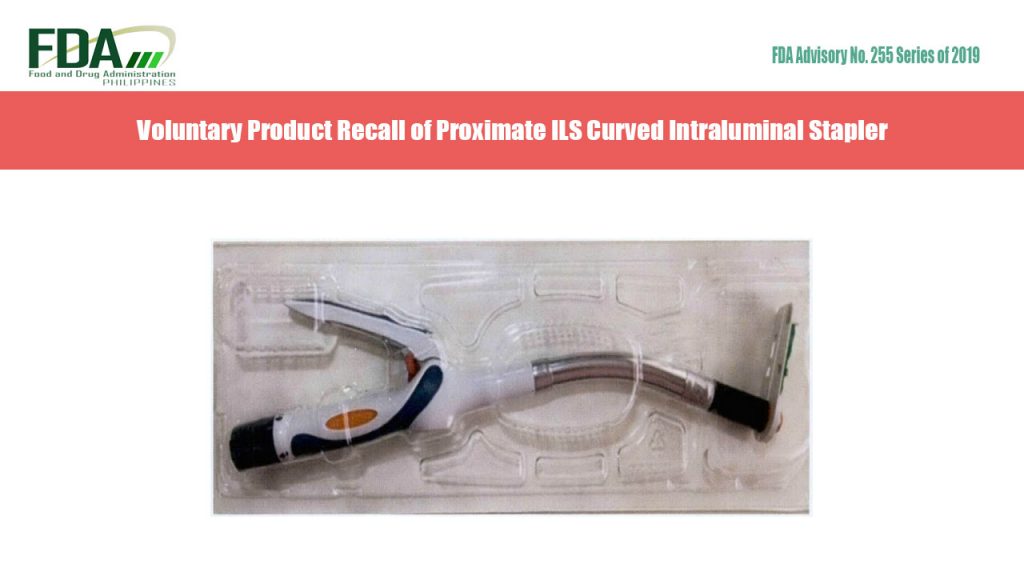
The Food and Drug Administration (FDA) warns all healthcare professionals and establishments on the voluntary recall of affected lots of Proximate ILS Curved Intraluminal Stapler with DVR No. 4981, manufactured […]
FDA Advisory No. 2019-254 || Public Health Warning Against the Purchase and Use of the Medical Device “INTROCAN SAFETY IV CANNULA WITH FIXATION WING (STERILE)” with the following Unregistered Article Numbers:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public against the purchase and use of the medical device with the following unregistered article numbers: SIZE UNREGISTERED […]
VENUE FOR UNIFIED LICENSING SEMINAR (ULS –RX) ON 29 AUGUST 2019

Please be informed that the venue of the Unified Licensing Seminar (ULS – RXI) on 29 August 2019 will be in PLAZA ALEMANIA, CORNER B. S. ONG STREET & GREGORIO T. LLUCH […]
FDA Advisory No. 2019-252 || Phased implementation of Administrative Order 2019-0007: “Revised Rules and Regulations on Electronic Nicotine and Non-Nicotine Delivery System (ENDS/ENNDS)”

The Food and Drug Administration (FDA) informs the consuming public and all stakeholders in the electronic cigarette industry that Administrative Order 2019-0007: “Revised Rules and Regulations on Electronic Nicotine and […]
PRESS RELEASE 22 August 2019: Warning retailers on promoting and selling of alcoholic beverages to minors (below 18 years old)

The Department of Health – Food and Drug Administration released recently an advisory warning retailers on promoting and selling of alcoholic beverages to minors (below 18 years old). This is […]
VENUE FOR QCCRR-MIN ON 29-30 AUGUST 2019 AND ULS-RX ON 30 AUGUST 2019

Please be informed that the venue of the Center for Cosmetics Regulation and Research (QCCRR-Min) on 29-30 August 2019 and Unified Licensing Seminar (ULS – RX) on 30 August 2019 […]



