FDA Advisory No. 2020-1986 || Safety Communication: Updates on Influenza Vaccine

Read more:-> FDA Advisory No.2020-1986
FDA has released a total of 303 COVID – 19 Test Kits (106 – PCR based, 99 – Rapid Antibody, 65 – Immunoassay and 33 – Others)
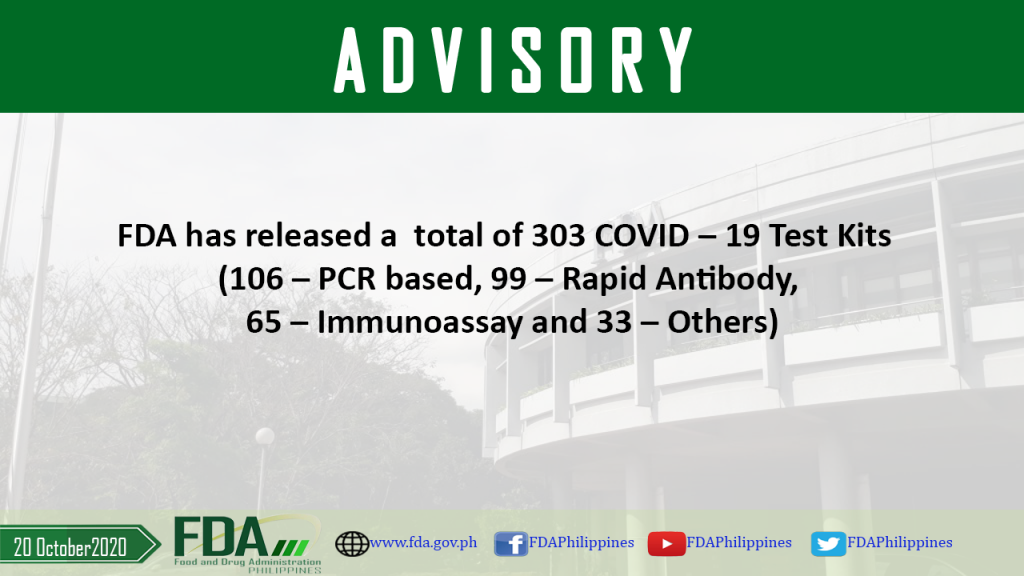
As of 30 October 2020, FDA has released a total of 303 COVID – 19 Test Kits (106 – PCR based, 99 – Rapid Antibody, 65 – Immunoassay and 33 […]
FDA Advisory No. 2020-128-A || Lifting the Advisory on the Registered Food Supplement PURITAN’S PRIDE ABC PLUS SENIOR MULTI DIETARY SUPPLEMENT under FDA Advisory No. 2020-128 with Subject” Public Health Warning Against the Purchase and Consumption of Unregistered Food Supplement

Read more:-> FDA Advisory No.2020-128-A
FDA Advisory No. 2020-1955 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “ZOCN DISPOSABLE FACE MASK FOR ADULT”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: The FDA verified through post-marketing surveillance […]
Guidelines on Compliance with Section 35 (b) of Republic Act No. 11223 (Universal Health Care Act) by All Drug, Medical Device, Biological and Medical Supplies Manufacturers to Submit Reports on Disclosure of Financial Relationships with Health Care Providers and Health Care Professionals

All comments can be sent at [email protected]. BACKGROUND The 1987 Philippine Constitution declares that it is the policy of the State to protect and promote the right to health of the […]
FDA Advisory No. 2020-1951 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic SECURE ALL IN ONE SKIN SUPPLEMENT BATH SOAP

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, SECURE ALL IN ONE SKIN SUPPLEMENT BATH SOAP . The abovementioned product was […]
FDA Advisory No. 2020-1950 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic LOVALI EXCELLENT VARSACE EROS EAU DE PARFUM NATYRAL SPRAY VAPORISATEUR (NO. 6156-17)

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, LOVALI EXCELLENT VARSACE EROS EAU DE PARFUM NATYRAL SPRAY VAPORISATEUR (NO. 6156-17). The […]
FDA Advisory No. 2020-1949 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic LOVALI EXCELLENT INVITATION VIP POUR HOME 1 MILLION DEODORANT ROLLON/EFFECTIVE 24H (NO. 6015-1)

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, LOVALI EXCELLENT INVITATION VIP POUR HOME 1 MILLION DEODORANT ROLLON/EFFECTIVE 24H (NO. 6015-1). […]
FDA Advisory No. 2020-1948 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic DAISO JAPAN EYEBROW GEL (CLEAR)
The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, DAISO JAPAN EYEBROW GEL (CLEAR). The abovementioned product was verified by FDA through […]
FDA Advisory No. 2020-1947 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic LA ‘SEINE SKIN CARE SERIES PAPAYA BODY BAR

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, LA ‘SEINE SKIN CARE SERIES PAPAYA BODY BAR. The abovementioned product was verified […]



