FDA Advisory No. 2020-2136 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food products: 1. SHAMROCK Sultana Raisins 2. GSJ PEANUTS Skinless […]
FDA Advisory No. 2020-2135 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food products: 1. WEILONG KISS BURN A Spicy Dried Tofu […]
FDA Advisory No. 2020-2134 ||Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food products: 1. NIU CHE SHUI Nuts in Yellow and […]
FDA Advisory No. 2020-2133 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food products: 1. WEILONG KISS BURN B Spicy Dried Tofu […]
Information, Education and Communication (IEC) Materials Relative to the Licensing of Household/Urban Hazardous Substances (HUHS) Establishments Covered Under Administrative Order No. 2019-0019 and FDA Circular No. 2020-025

In the interest of the service, the Food and Drug Administration (FDA) herewith provides, for information and guidance of all concerned stakeholders, a collated Frequently Asked Questions (FAQs) relative to […]
Draft for Comment of Application for Labeling Compliance of Drug Products under Maximum Drug Retail Price

All comments can be sent at [email protected]. Deadline of submission of comments shall be by 08 January 2021. I. BACKGROUND/RATIONALE Rule 31, Section 1 of the Joint DOH-DTI-IPO-BFAD Administrative Order […]
FDA has released a total of 335 COVID – 19 Test Kits (119 – PCR based, 104 – Rapid Antibody, 68 – Immunoassay and 44 – Others).
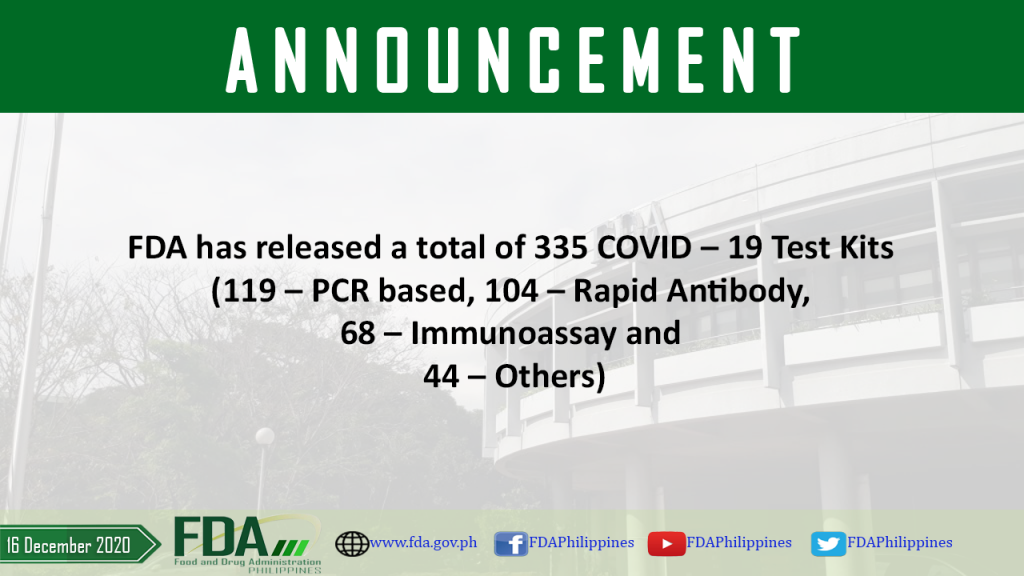
As of 16 December 2020 , FDA has released a total of 335 COVID – 19 Test Kits (119 – PCR based, 104 – Rapid Antibody, 68 – Immunoassay and […]
FDA Circular No. 2020-037 || Reissuance of the Guidelines on the Implementation of the Use of Electronic Means of Prescription for Drugs for the Benefit of Individuals Vulnerable to COVID-19
I. RATIONALE Last 17 March 2020, the Food and Drug Administration issued FDA Circular No. 2020-007 entitled “Guidelines on the Implementation of the Use of Electronic Means of Prescription for […]
FDA Circular No. 2020-028-A || EXTENSION OF THE EFFECTIVITY OF FDA CIRCULAR NO. 2020-028 ENTITLED “REISSUANCE OF THE GUIDELINES FOR THE REGISTRATION OF DRUG PRODUCTS UNDER EMERGENCY USE (DEU) FOR THE CORONAVIRUS DISEASE 2019 (COVID-19)”
In the interest of service and due to the continuing COVID-19 Pandemic, the effectivity of FDA Circular No. 2020-028 entitled “Reissuance of the Guidelines for the Registration of Drug Products […]
FDA Circular No. 2020-022-A || EXTENSION OF THE EFFECTIVITY OF FDA CIRCULAR NO. 2020-022 ENTITLED “REISSUANCE OF THE GUIDELINES ON THE IDENTIFICATION, NOTIFICATION, EVALUATION, REGULATORY ENFORCEMENT ACTION, AND REVIEW AND MONITORING OF DONATED HEALTH PRODUCTS SOLELY INTENDED TO ADDRESS COVID-19 PUBLIC HEALTH EMERGENCY”
In the interest of service and due to the continuing COVID-19 Pandemic, the effectivity of FDA Circular No. 2020-022 entitled “Reissuance of the Guidelines on the Identification, Notification, Evaluation, Regulatory […]



