FDA Advisory No.2021-0513 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic SANLONG HAND SANITIZER
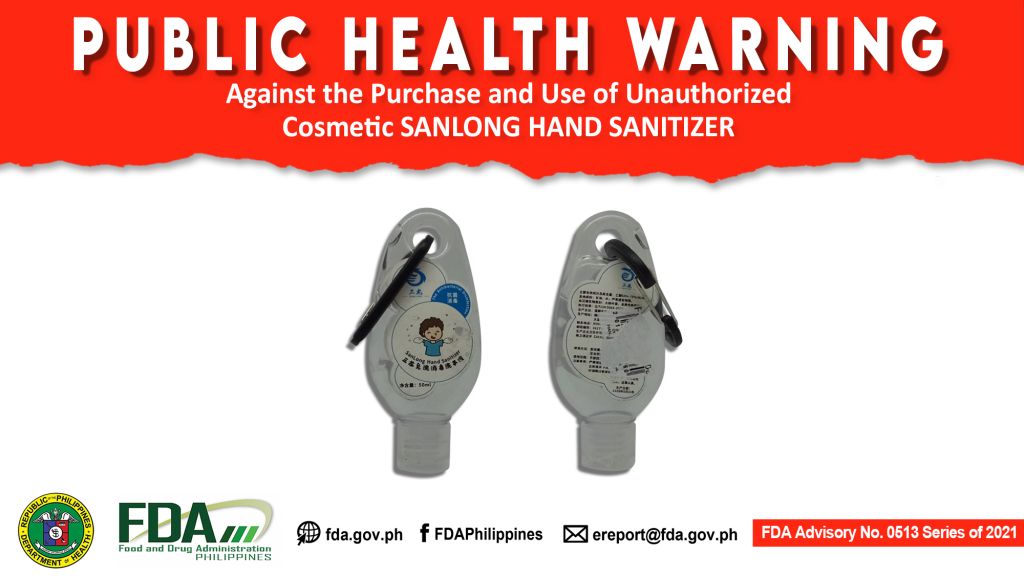
The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product, SANLONG HAND SANITIZER . The abovementioned product was verified by FDA through postmarketing […]
FDA Advisory No.2021-0062-A || Lifting the Advisory on the Notified Cosmetic Product “VITAKERATIN EXPERT SALON TREATMENT BRAZILIAN STRAIGHT FOR FRIZZY & UNRULY HAIR” under FDA Advisory No. 2021-0062 “Public Health Warning Against the Purchase and Use of Non-Compliant Cosmetic Product Vitakeratin Expert Salon Treatment Brazilian Straight For Frizzy & Unruly Hair” Dated 14 January 2021

The Food and Drug Administration (FDA) informs the public that the Cosmetic product VITAKERATIN EXPERT SALON TREATMENT BRAZILIAN STRAIGHT FOR FRIZZY & UNRULY HAIR with Notification No. 1000007428660 is a […]
FDA Advisory No.2020-1312-A || Lifting the Advisory on the Notified Product “KEDMA EYE CREAM” under FDA Advisory No. 2020-1312 “Public Health Warning Against the Purchase and Use of Unnotified Cosmetic KEDMA EYE CREAM” Dated 06 July 2020

The Food and Drug Administration (FDA) informs the public that the Cosmetic product KEDMA EYE CREAM with Notification No. 1000006677683 has been notified by the Market Authorization Holder, F Cosmetics […]
FDA Advisory No.2020-577-A || Lifting the Advisory on the Notified Cosmetic Product “SUNBRIGHT SERIES KERATIN & COLLAGEN HAIR COLOR PERMANENT HAIR COLOR (7/45 FLOWERINESS RED)” under FDA Advisory No. 2020-577 “Public Health Warning Against the Purchase and Use of Unnotified Cosmetic SUNBRIGHT SERIES KERATIN & COLLAGEN HAIR COLOR PERMANENT HAIR COLOR (7/45 FLOWERINESS RED)” Dated 08 April 2020
The Food and Drug Administration (FDA) informs the public that the Cosmetic product SUNBRIGHT SERIES KERATIN & COLLAGEN HAIR COLOR PERMANENT HAIR COLOR (7/45 FLOWERINESS RED) with Notification No. 1000003258038 […]
FDA Advisory No.2021-0497 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic Product “BREYLEE PORE MINIMIZER SERUM”

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product BREYLEE PORE MINIMIZER SERUM. The abovementioned product was verified by FDA through post […]
FDA Advisory No.2021-0496 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic Product “PEIXEN CLASSIC RED WINE EYEBROW GEL”

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product PEIXEN CLASSIC RED WINE EYEBROW GEL. The abovementioned product was verified by FDA […]
FDA Advisory No.2021-0495 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic Product “OH! HELLO LASHES! ALOE VERA LASH & BROW SERUM”
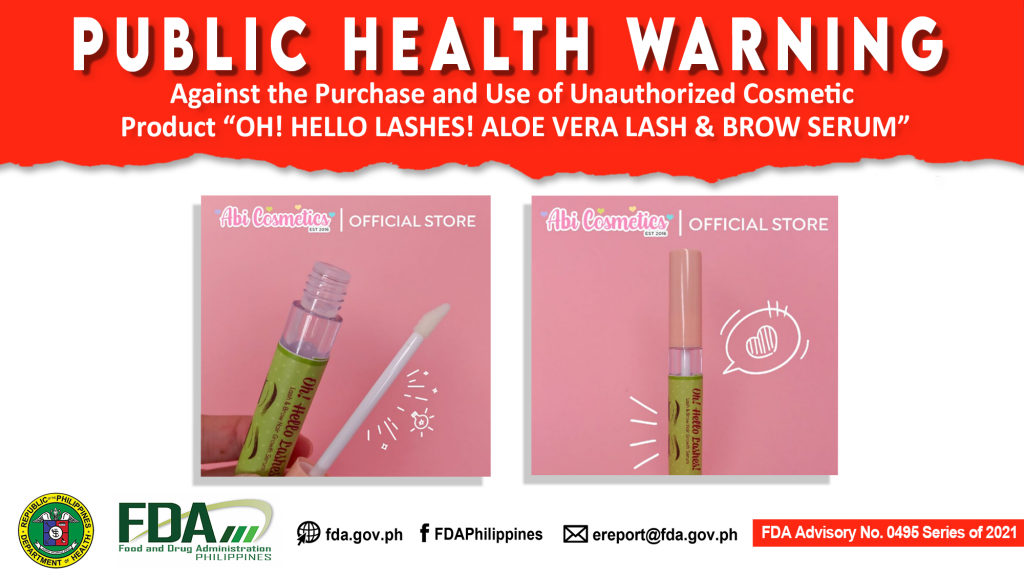
The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product OH! HELLO LASHES! ALOE VERA LASH & BROW SERUM. The abovementioned product was […]
FDA Advisory No.2021-0494 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic Product “MEGAWISE PETROLEUM JELLY”

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product MEGAWISE PETROLEUM JELLY. The abovementioned product was verified by FDA through post marketing […]
FDA Advisory No.2021-0493 || Public Health Warning Against the Purchase and Use of Unauthorized Cosmetic Product “CARSLAN REPAIR SERUM FOUNDATION”

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized cosmetic product “CARSLAN REPAIR SERUM FOUNDATION”. The abovementioned product was verified by FDA through post […]
FDA Advisory No.2021-0492 || Public Health Warning Against the Purchase and Use of Unauthorized Household/Urban Pesticide (H/UP) SHAN JIA PAI 0.18% INSECTICIDE

The Food and Drug Administration (FDA) warns the public from purchasing and using the unauthorized household/urban pesticide (H/UP) product, SHAN JIA PAI 0.18% INSECTICIDE. The abovementioned product was verified by […]



