FDA Advisory No.2024-0384 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “USHAS LIP MASK (GRAPE)”
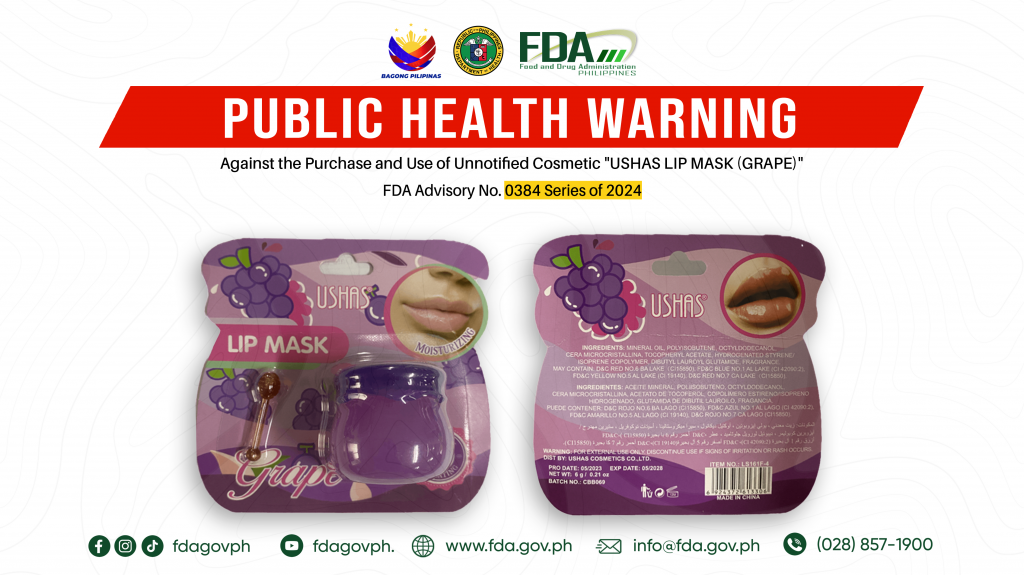
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, USHAS LIP MASK (GRAPE). The abovementioned product was verified by FDA through postmarketing […]
FDA Advisory No.2024-0382 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “POND’S WHITE BEAUTY SPOT-LESS ROSY WHITE DAILY FACIAL FOAM”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, POND’S WHITE BEAUTY SPOT-LESS ROSY WHITE DAILY FACIAL FOAM. The abovementioned product was […]
FDA Advisory No.2024-0381 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “BEBETA BABY NATURAL TOOTH GEL WITH XYLITOL FLUORIDE FREE (STRAWBERRY FLAVOR)”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, BEBETA BABY NATURAL TOOTH GEL WITH XYLITOL FLUORIDE FREE (STRAWBERRY FLAVOR). The abovementioned […]
FDA Advisory No.2024-0380 || Public Health Warning Against the Purchase and Use of Unnotified Toy and Childcare Article (TCCA) “BEBETA FREE FLOW TRAINING CUP 8OZ/235ML”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified toy and childcare article (TCCA) product, BEBETA FREE FLOW TRAINING CUP 8OZ/235ML. The abovementioned product […]
FDA Advisory No.2024-0378 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “GS HAIR BOTOX UNIQUE BRAZILIAN STRAIGHT SUNFLOWER”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, GS HAIR BOTOX UNIQUE BRAZILIAN STRAIGHT SUNFLOWER. The abovementioned product was verified by […]
FDA Advisory No.2024-0377 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “JOCO NATURAL LIPS NATURAL LIP BALM & LIP MOISTURIZER”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, JOCO NATURAL LIPS NATURAL LIP BALM & LIP MOISTURIZER. The abovementioned product was […]
FDA Advisory No.2024-0376 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “PRODUCT IS IN FOREIGN LANGUAGE”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, PRODUCT IS IN FOREIGN LANGUAGE. The abovementioned product was verified by FDA through […]
FDA Advisory No.2024-0375 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “GREENIKA GLUTA-BLEACH”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, GREENIKA GLUTA-BLEACH. The abovementioned product was verified by FDA through postmarketing surveillance and […]
FDA Advisory No.2024-0374 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “LADY ROMANCE WHITE MINERAL OIL”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, LADY ROMANCE WHITE MINERAL OIL. The abovementioned product was verified by FDA through […]
FDA Advisory No.2024-0373 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “ROSMAR SKIN ESSENTIALS 3 IN 1 UWU! AHA | BHA | PHA 24 HOURS SERUM”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, ROSMAR SKIN ESSENTIALS 3 IN 1 UWU! AHA | BHA | PHA 24 […]



