FDA Advisory No.2022-0107-A || Lifting of the FDA Advisory No. 2022-0107 entitled “Reiteration on Prohibition of Online Selling of FDA Certified COVID-19 Test Kits including Self-Administered COVID-19 Test Kits”

In line with the Department of Health Department Memorandum (DM) No. 2022-0033 entitled “Guidelines on the Use of Self-Administered Antigen Testing for COVID-19, the Food and Drug Administration (FDA) informs […]
FDA Advisory No.2022-1519 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “SANSURE BIOTECH SARS-COV-2-ANTIGEN TEST CASSETTE”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. SANSURE BIOTECH SARS-COV-2-ANTIGEN TEST CASSETTE […]
FDA Advisory No.2022-1251 || Public Health Warning Against the Purchase and Use of the Uncertified Self-Administered COVID-19 Test Kit “LEADINBIO COVID-19 ANTIGEN RAPID TEST KIT (SWAB)”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified self-administered COVID-19 test kit: 1. LEADINBIO COVID-19 ANTIGEN RAPID […]
FDA Advisory No.2022-0675 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “CORE TESTS® COVID-19 SALIVA AG TEST”
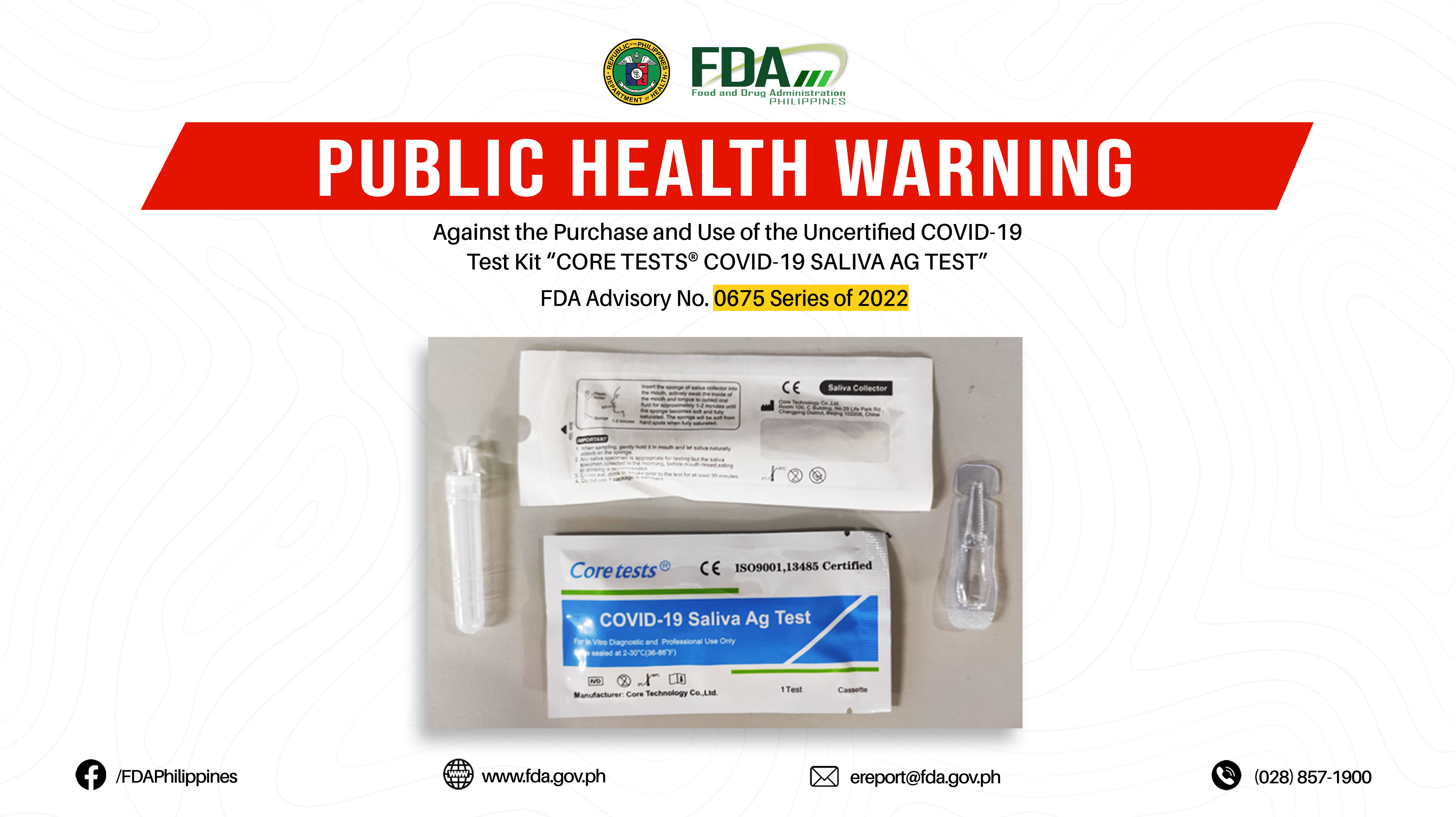
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. CORE TESTS® COVID-19 SALIVA AG […]
FDA Advisory No.2022-0623 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “VIVADIAG PRO SARS-COV-2 AG RAPID TEST”
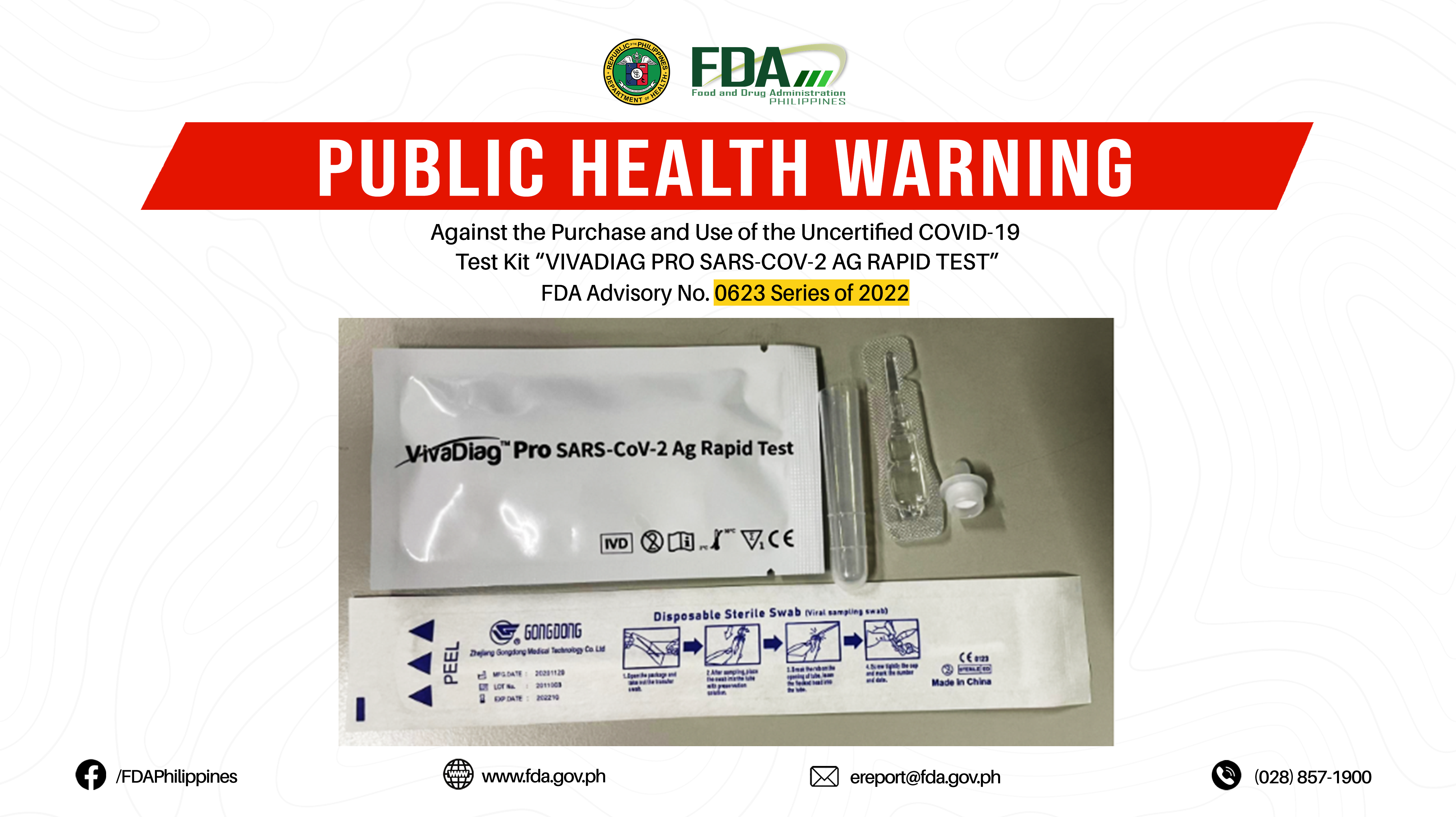
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. VIVADIAG PRO SARS-COV-2 AG RAPID […]
FDA Advisory No.2022-0607 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “SARS-COV-2 AG RAPID TEST”
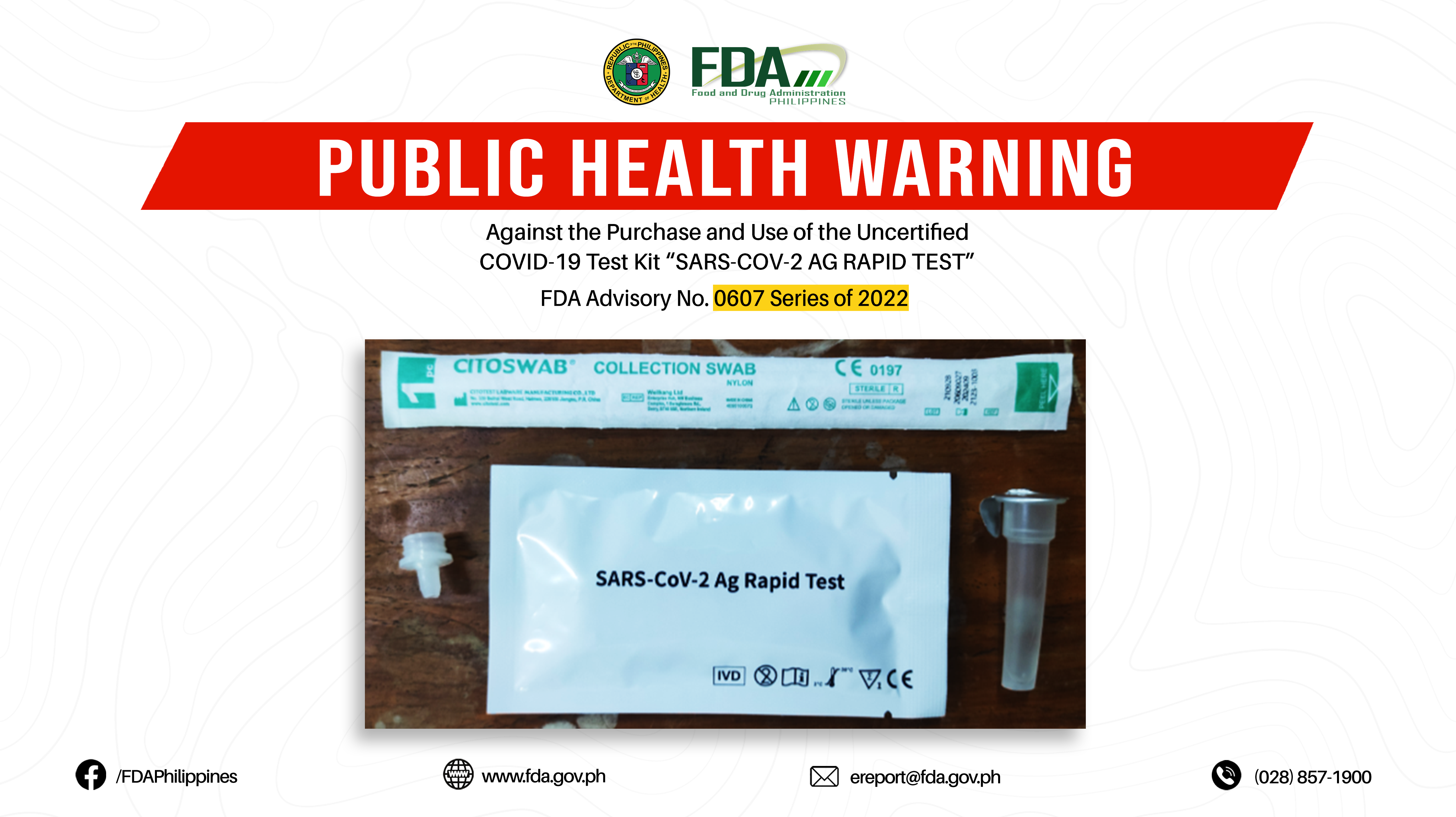
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. SARS-COV-2 AG RAPID TEST The […]
FDA Advisory No.2022-0609 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “PARTNERS COVID-19 IGG/IGM RAPID TEST CASSETTE (WB/S/P)”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. PARTNERS COVID-19 IGG/IGM RAPID TEST […]
FDA Advisory No.2022-0604 || Public Health Warning on Falsified Drug Product “DESREM” Confirmed by the World Health Organization (WHO)

The Food and Drug Administration (FDA) notifies the public on the WHO Medical Product Alert on falsified Remdesivir 100 mg/vial Lyophilized Powder for Injection for IV Infusion with brand name […]
FDA Advisory No.2022-0052-A || Lifting of the FDA Advisory No. 2022-0052 entitled “Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “HEALGEN® CORONAVIRUS AG RAPID TEST CASSETTE (SWAB)”
The Food and Drug Administration (FDA) informs all healthcare professionals and the general public that the advisory on Healgen® Coronavirus Ag Rapid Test Cassette (Swab) manufactured by Healgen Scientific Limited […]
FDA Advisory No.2022-0539 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “PARTNERS COVID-19 ANTIGEN RAPID TEST CASSETTE (NASAL SWAB)”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the uncertified COVID-19 test kit: 1. PARTNERS COVID-19 ANTIGEN RAPID TEST […]



