FDA Advisory No.2024-0568 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “LIVINGSTONE HOT AND COLD PACK REUSABLE”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. LIVINGSTONE HOT AND COLD PACK […]
FDA Advisory No.2024-0567 || Public Health Warning Against the Purchase and Use of the Following Unnotified FELDON PREMIER HAND INSTRUMENTS Medical Device Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device products: 1. “LIGATURE DIRECTOR TUCKER ORTHO” 2. […]
FDA Advisory No.2024-0566 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “STERILAB CEVICAL RAMBRUSH, MF207-P22-3”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. STERILAB CEVICAL RAMBRUSH, MF207-P22-3 The […]
FDA Advisory No.2024-0565 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “MEDLINE MINI TRACHEOSTOMY CARE KIT”
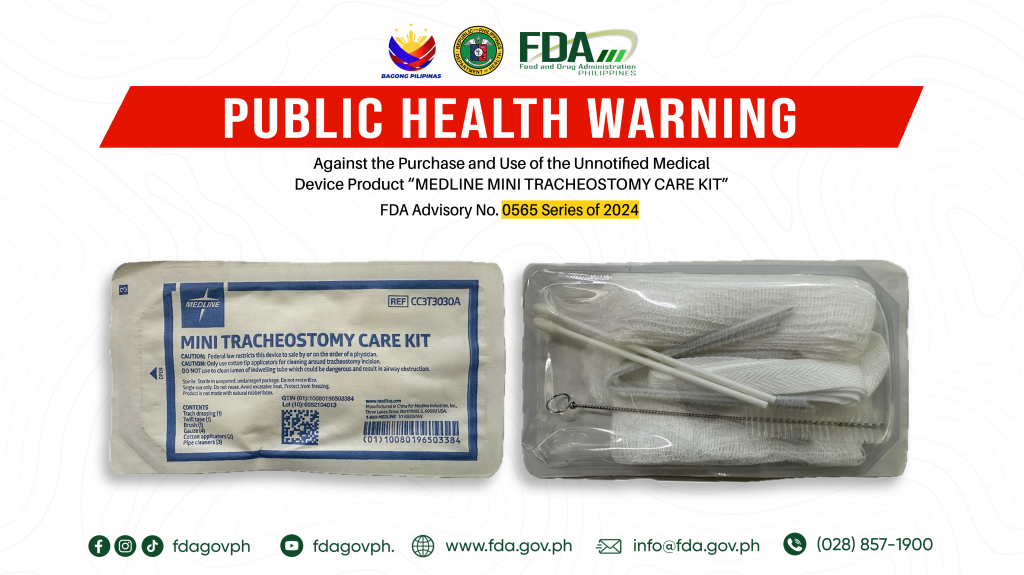
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. MEDLINE MINI TRACHEOSTOMY CARE KIT […]
FDA Advisory No.2024-0564 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “UNIMEX® WOODEN TONGUE DEPRESSOR”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. UNIMEX® WOODEN TONGUE DEPRESSOR The […]
FDA Advisory No.2024-0563 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Product “PRO HEALTH CARE NEBULIZER KIT”
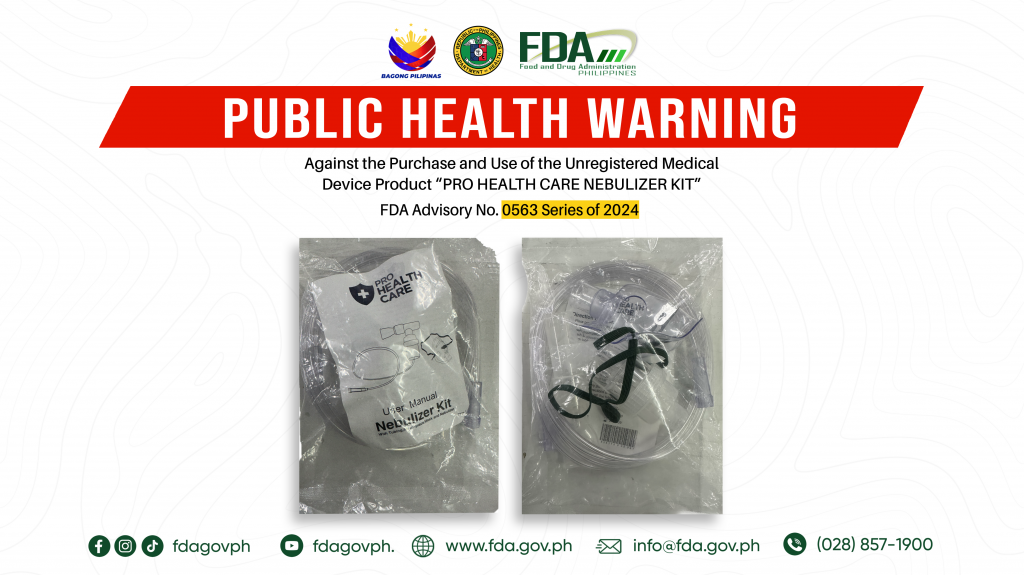
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product: 1. PRO HEALTH CARE NEBULIZER KIT […]
FDA Advisory No.2024-0562 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “LANCING DEVICE, MODEL: HH-X-T”
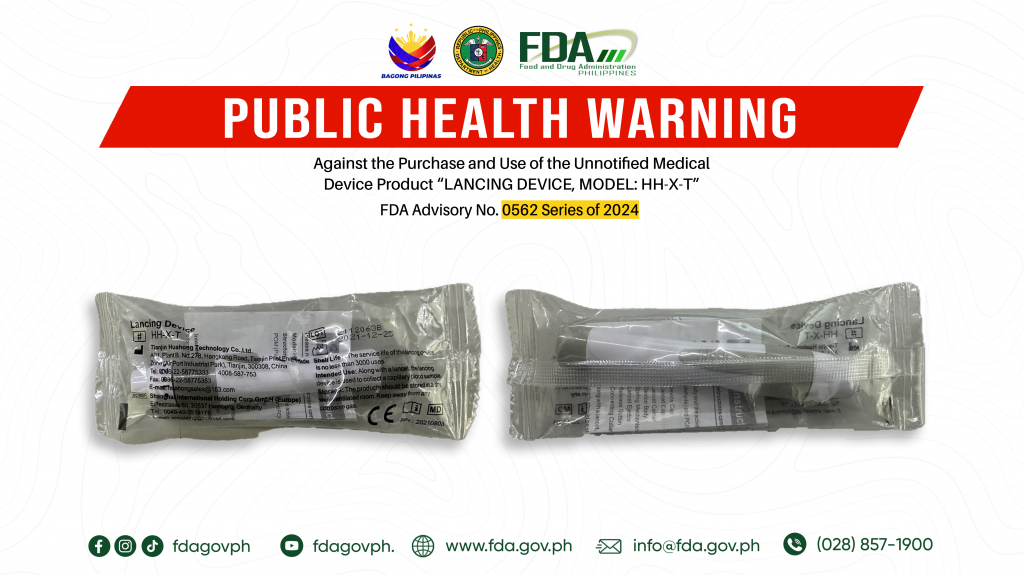
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. LANCING DEVICE, MODEL: HH-X-T The […]
FDA Advisory No.2024-0561 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “WELCHALLYN KLEENSPEC INSPECT SPECULUM”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. WELCHALLYN KLEENSPEC INSPECT SPECULUM The […]
FDA Advisory No.2024-0560 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “VAGINAL SPECULUM”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. VAGINAL SPECULUM The FDA verified […]
FDA Advisory No.2024-0559 || Public Health Warning Against the Purchase and Use of the Medical Device Product “DIGITAL THERMOMETER”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product: 1. DIGITAL THERMOMETER The FDA verified […]



