FDA Advisory No.2024-0576 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. OTC Clindamycin Phosphate Gel 2. OTC Z36021451 [Label in […]
FDA Advisory No.2024-0575-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Hindi Rehistradong Gamot:
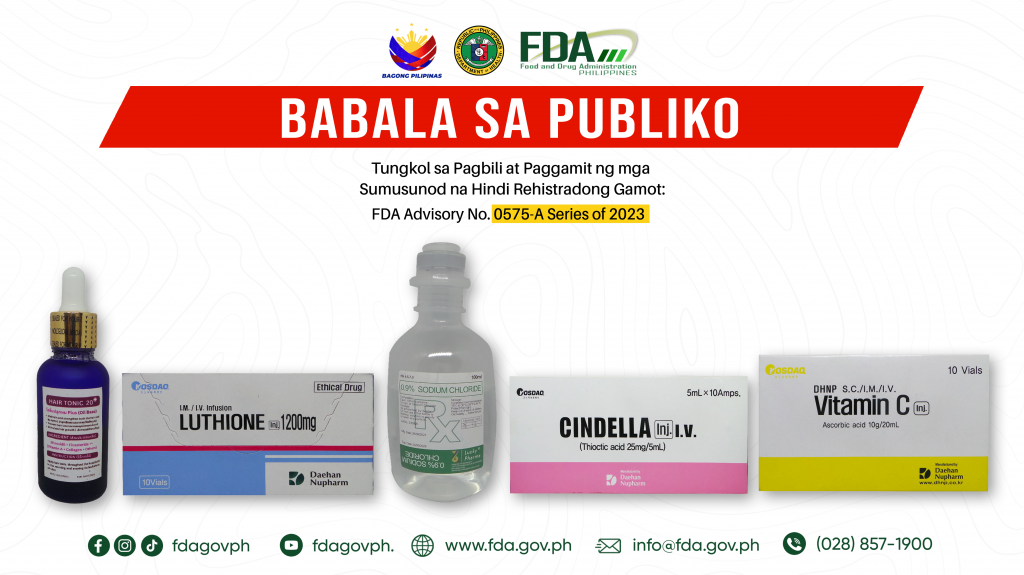
The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. Hair Tonic 20 Minoxidil + Finasteride ++ 2. KOSDAQ […]
FDA Advisory No.2024-0575 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. Hair Tonic 20 Minoxidil + Finasteride ++ (Vitamin A […]
FDA Advisory No.2024-0574-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Hindi Rehistradong Gamot:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga sumusunod na hindi rehistradong gamot: 1. OTC Ibuprofen Granules 2. Zang Jikang® (as translated) […]
FDA Advisory No.2024-0574 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. OTC Ibuprofen Granules 2. Zang Jikang® (as translated) [Label […]
FDA Advisory No.2024-0557-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Hindi Rehistradong Gamot:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga sumusunod na hindi rehistradong gamot: 1. OTC Sodium Cromoglicate Eye Drops 2. Ribavirin Eye […]
FDA Advisory No.2024-0557 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. OTC Sodium Cromoglicate Eye Drops 2. Ribavirin Eye Drops […]
FDA Advisory No.2024-0536-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Hindi Rehistradong Gamot:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga sumusunod na hindi rehistradong gamot: 1. OTC KAIXIU Fenghan Ganmao Keli 2. Qian Jiang […]
FDA Advisory No.2024-0536 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: 1. OTC KAIXIU® Fenghan Ganmao Keli 2. Qian Jiang Ji […]
FDA Advisory No.2024-0535-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Hindi Rehistradong Gamot:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga sumusunod na hindi rehistradong gamot: 1. NSAIDs Dexirofen (Dexibuprofen) Tablet 2. Saxenda® 3mL x […]



