FDA Advisory No.2022-1535 || WARNING AGAINST THE FALSIFICATION/ TAMPERING OF CASH DEPOSIT SLIP AND OTHER RELATED DOCUMENTS SUBMITTED TO THE FDA

The Food and Drug Administration (FDA) – FDA Academy has recently become aware of acts involving tampered/altered/falsified Cash Deposit Slips submitted by participant stakeholders as proof of payment for training/s […]
FDA Advisory No.2022-1291-A || Hindi Awtorisado at Lantarang Pag-Gamit ng FDA Advisory Template sa Pag-Rekomenda ng Produktong Pangkalusugan
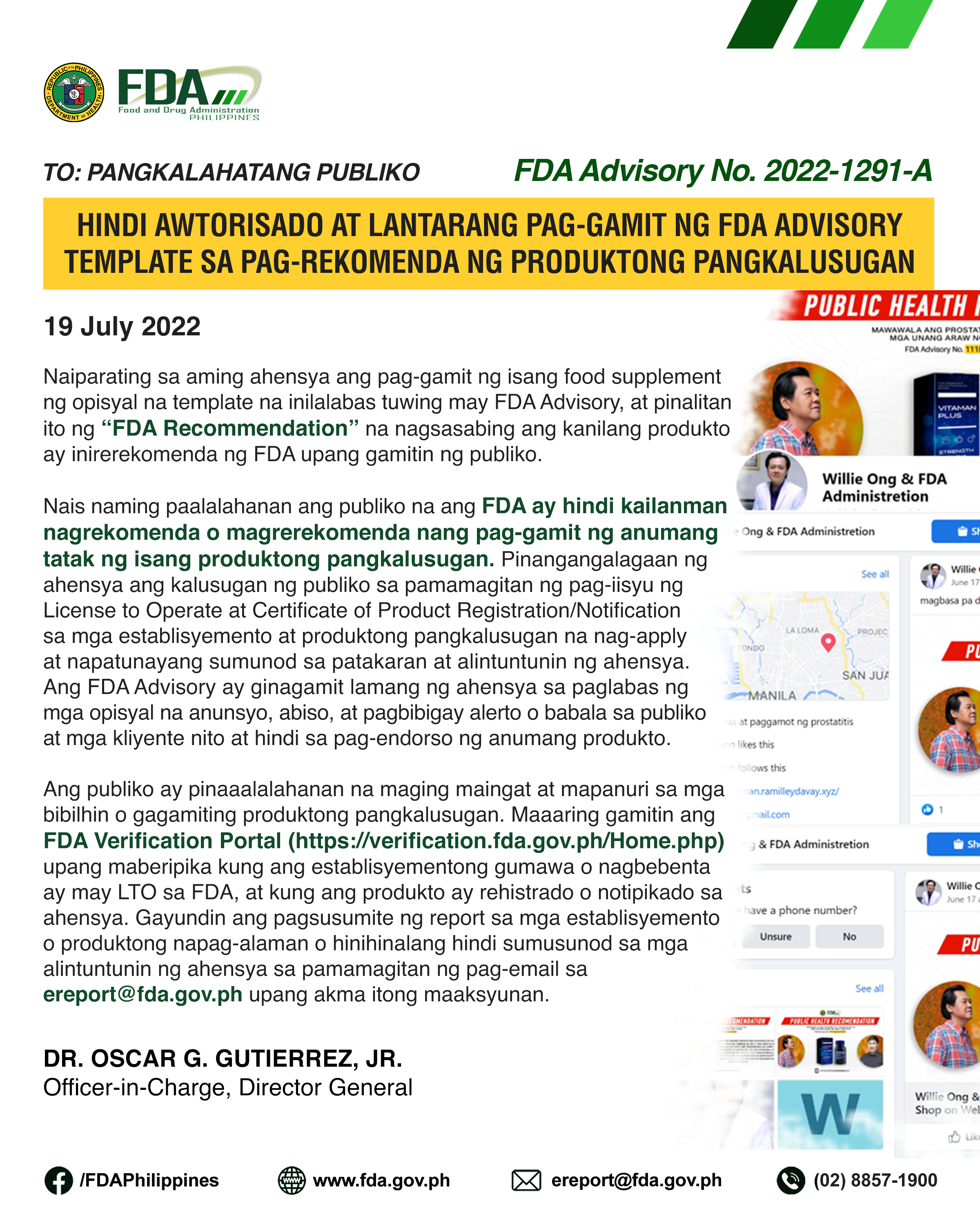
Naiparating sa aming ahensya ang pag-gamit ng isang food supplement ng opisyal na template na inilalabas tuwing may FDA Advisory, at pinalitan ito ng “FDA Recommendation” na nagsasabing ang kanilang […]
FDA Advisory No.2022-1291 || Unauthorized Use of the FDA Advisory Template in the Recommendation of a Health Product
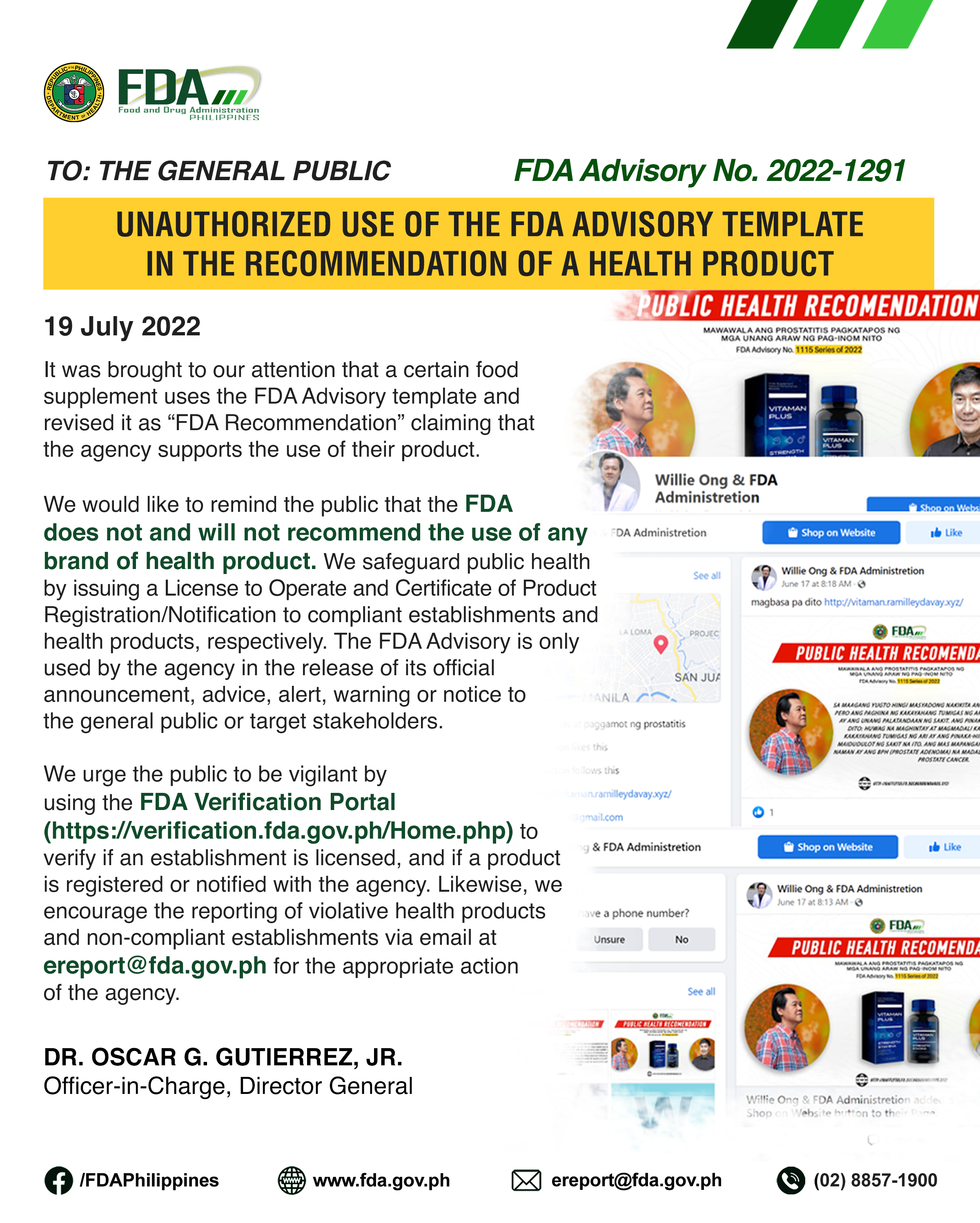
It was brought to our attention that a certain food supplement uses the FDA Advisory template and revised it as “FDA Recommendation” claiming that the agency supports the use of […]
FDA Advisory || CLARIFICATION ON THE SAFETY OF ALL LOCALLY MANUFACTURED LUCKY ME! INSTANT NOODLE

The FDA wishes to clarify that all flavor variants of locally manufactured Lucky Me! Instant Noodle, namely Pancit Canton Regular, Pancit Canton Extra Hot Chili, Pancit Canton Chilimansi, and Instant […]
FDA Advisory No.2022-1293 || UPDATES ON ETHYLENE OXIDE IN LUCKY ME! INSTANT NOODLES

Several countries in the European Union have alerted the presence of ethylene oxide in Lucky Me! Brand Noodle Products. Ethylene oxide is used as treatment against the microbiological contamination of […]
FDA Advisory No.2022-1252 || FDA STATEMENT ON THE ISSUE OF RECALLED FOOD PRODUCTS DUE TO THE PRESENCE OF ETHYLENE OXIDE

The Food and Drug Administration Philippines has received reports on the on-going recall of certain batches of the product “Lucky Me! Instant Pancit Canton Noodles” in European countries and Taiwan […]
FDA Advisory No.2022-1222 || CAUTION ON ENGAGING THE SERVICES OF “JOHANNA CARDONA” AND OTHER ENTITIES CLAIMING TO EXPEDITE THE ISSUANCE OF MARKET AUTHORIZATION APPLICATIONS
The Food and Drug Administration (FDA) reiterates that transactions with the Agency should only be through official channels. We have received a report from a concerned citizen that a certain […]
FDA Advisory No.2022-1118 || Caution on Engaging the Services of “Abegail Rico” and other Entities Claiming to be Agents of the FDA

The Food and Drug Administration (FDA) strongly advises the public to deal only through official channels when transacting with the Agency. Through the indorsement of the Department of Trade and […]
FDA Advisory || Relative to the celebration of FDA’s Sportsfest CY 2022, telephone service of the Food and Drug Action Center (FDAC) will be temporarily suspended on 03 June 2022, Friday.

Relative to the celebration of FDA’s Sportsfest CY 2022, telephone service of the Food and Drug Action Center (FDAC) will be temporarily suspended on 03 June 2022, Friday. However, receiving […]
FDA Advisory No.2022-1090 || Frequently-Asked Questions (FAQs) on FDA Circular (FC) No. 2022-001 “Repealing FDA Circular No. 2021-004 “Revised Interim Guidelines for the Issuance of License to Operate (LTO) and Certificate of Product Notification (CPN) for Manufacturers, Distributors and Traders of Rubbing Alcohol Products Under the Center for Cosmetics and Household/Urban Hazardous Substances Regulation and Research” dated 09 February 2021”

On 31 March 2022, the Food and Drug Administration (FDA) issued FDA Circular (FC) No. 2022-001 “Repealing FDA Circular No. 2021-004 “Revised Interim Guidelines for the Issuance of License to […]



