You may submit your comments in MSWord format to [email protected]. The deadline for submission of comments is on 02 February 2023.
I. RATIONALE
It is a policy of the State as embodied in Article II, Section 15 of the 1987 Constitution to protect and promote the right to health of the people and instill health consciousness among them and in Section 12, Article XIII of the 1987 Constitution to establish and maintain an effective food and drug regulatory system and undertake appropriate health manpower development and research, responsive to the country’s health needs and problems.
It is further declared as a policy of the State under Republic Act No. 7394 or the Consumer Act of the Philippines to enforce compulsory labelling, and fair packaging to enable the consumer to obtain accurate information as to the nature, quality and quantity of the contents of consumer products and to facilitate comparison of the value of such products.
As the 2002 Recommended Energy and Nutrient Intake (RENI) needs updating, the Department of Science and Technology – Food and Nutrition Research Institute (DOST-FNRI) has formulated and developed set of dietary standards based on the recent and updated available information for the following: 1) Estimated Average Requirement (EAR); 2) Recommended Energy Intake/Recommended Nutrient Intake (REI/RNI); 3) Adequate Intake (AI); 4) Tolerable Upper Intake/Upper Limit (UL); and 5) Acceptable Macronutrient Distribution Range (AMDR), which are now prescribed in the 2015 Philippine Dietary Reference Intakes (PDRI).
In line with these changes, the Food and Drug Administration (FDA) shall use the new REI/RNI prescribed in the 2015 PDRI as the reference standard in nutrition labelling of processed food products, planning food fortification program, nutrition advocacy, and formulating laws, among others. The REI/RNI shall be used in place of the 2002 RENI for Filipinos. Thus, updating the Bureau Circular No.16 s.2005. The guidelines on the use of REI/RNI for nutrition information on the label of processed foods are promulgated under this Circular.
II. OBJECTIVES
This Circular aims to update the reference of dietary standard used in processed food products.
III. SCOPE
This Circular shall cover all nutrition labeling of processed food products, planning food fortification program, nutrition advocacy, and formulating laws, among others.
IV. GUIDELINES ON THE USE OF RECOMMENDED ENERGY/NUTRIENT INTAKE FOR LABEL DECLARATION OF NUTRITION INFORMATION
- % REI/RNI shall be used in the nutrition information table (Table 1) following the Recommended Energy/Nutrient Intake levels prescribed by FNRI. Tables 2-4 (See Annex A) summarize the reference values for computing energy and nutrient levels in nutrition
Table 1. Sample Format for Nutrition Facts Declaration
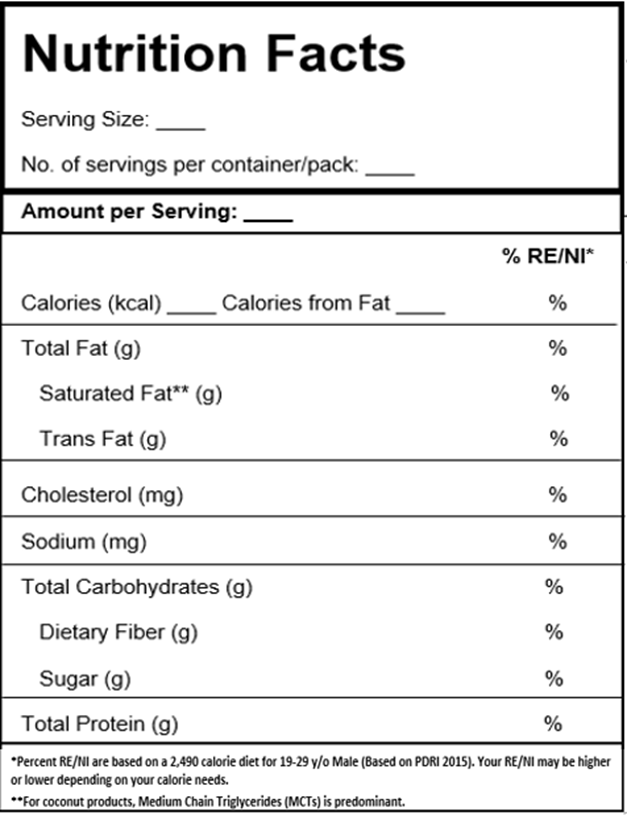
- The statement “Based on PDRI 2015” shall be declared below the Nutrition Facts/ Nutrition Information/ Nutritive Value table.
- For purposes of computing the nutrient content expressed in terms of % REI/RNI the computation shall be based on the PDRI 2015 for male adults ages nineteen (19) to twenty-nine (29). In cases of food products intended for a specific group, REI/RNI values for the said group shall be made as the basis of REI/RNI declaration and such fact shall be indicated on the label.
- When the product is intended for 2 or more age groups, age group with the highest values for energy and nutrients shall be used, i.e. products intended for 13-18 years old (13-15 and 16-18 y/o), recommended values for male, 16-18 years old shall be
- Computation of iron level for general population shall be based on iron values for male, unless the product is intended for female, the iron values for female shall be
- For the purpose of computation on the levels of fiber, the mid-value 23 g based on the recommended range 20-25 g for adult and the mean value 13 g based on the recommended range 11-14 g for children 6-9 y/o shall be
- Average amount of the actual values based on analysis shall be used to declare levels of energy and nutrients. However, other recognized nutrient database maybe used as basis for the computation as applicable. The amounts of energy and nutrients shall be declared in whole numbers. However, for B-vitamins, cholesterol, saturated fat and trans- fat, average values shall be declared without increment or rounding off, and on the nearest tenths place or hundredths place. Example, if the average amount is 0.32 mg cholesterol, declaration shall be 0.3 mg; if the average amount is 0.0088 mg, declaration shall be 0.01 mg.
- %REI/RNI values shall be declared in whole numbers. Nutrients present in amounts less than 2 percent of the RNI shall be indicated by the statement “contains less than (or symbol „<‟) 2% RNI” or by an asterisk referring to this
- Certain food products that contain insignificant amounts of nutrients to be declared on the label can be exempted from specific nutrient analysis based on its innate composition (e.g. dried mango with no saturated fat, cholesterol, trans- fat; coconut oil which does not have fiber, sodium, protein, ash/minerals; plant based single component product (zero [0] cholesterol), single component animal based product (zero [0] dietary fiber), coffee and most spices, flavor extracts, food color, as determined by FDA. The product that contains insignificant amount of specific nutrient, this particular nutrient shall be listed in the nutrition information and the amount shall be declared as “0”.
- The Tolerable Upper Intake Levels or Upper Limits (UL) (see Annex B) is the highest average daily nutrient intake level likely to pose no adverse health effects to almost all individuals in the general population. The nutrients may be obtained from different dietary sources such as food and supplements. Hence, UL shall not be used as basis for the maximum limit of vitamins and minerals for Food Supplement.
- Products such as Food for Special Medical Purposes, Food for Special Dietary Uses, Infant Formula, Milk Supplement, bottled water among others shall follow its own prescribed guidelines/standards in nutrition labelling.
V. TRANSITORY PROVISION
After 12 months from effectivity of this issuance, non-compliant products shall thereafter be deemed misbranded and appropriate sanctions against the violating establishment shall be imposed.
VI. REPEALING CLAUSE
The Bureau Circular No. 16 s. 2005 “Adopting the 2002 Recommended Energy and Nutrient Intakes as the New Dietary Standard”, and other FDA issuances inconsistent with this Circular are hereby repealed accordingly.
VII. SEPARABILITY CLAUSE
If any provision of this Circular be declared as invalid or unenforceable, the validity and enforceability of the remaining portions or provisions shall remain in full force and effect.
VIII. EFFECTIVITY
This Circular shall take effect fifteen (15) days after its publication in the Official Gazette or in any newspaper of general circulation and upon filing with the University of the Philippines Law Center Office of the National Administrative Register.



