Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng pekeng bersyon ng mga:
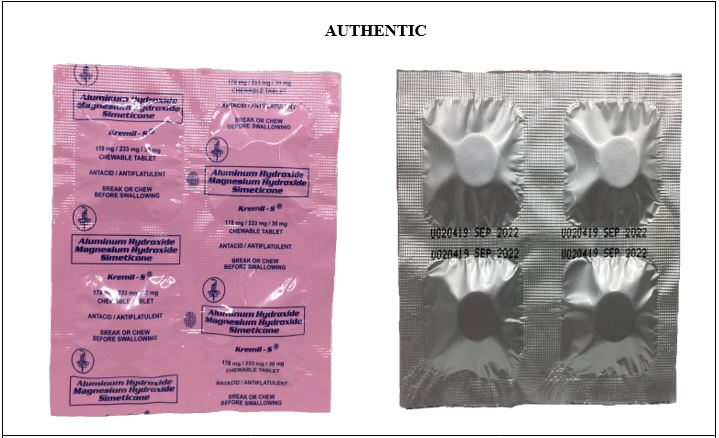
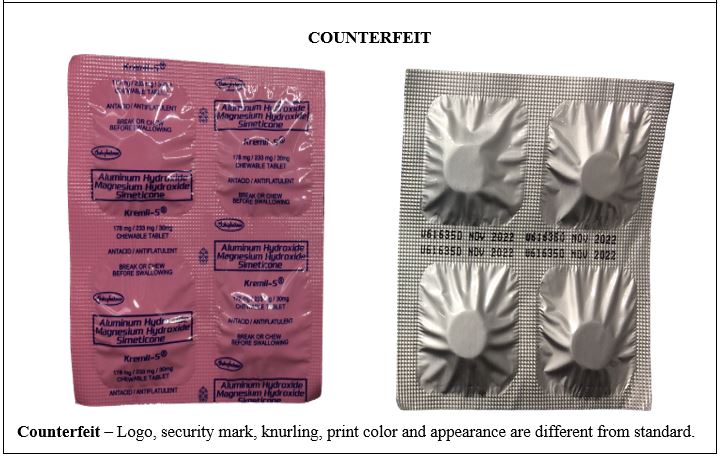
Figure 1. Comparison between the Authentic and Counterfeit Aluminum Hydroxide / Magnesium Hydroxide / Simeticone (Kremil-S®) 178mg/233mg/30mg Chewable Tablet (Lot Nos. U213457 and U616350)
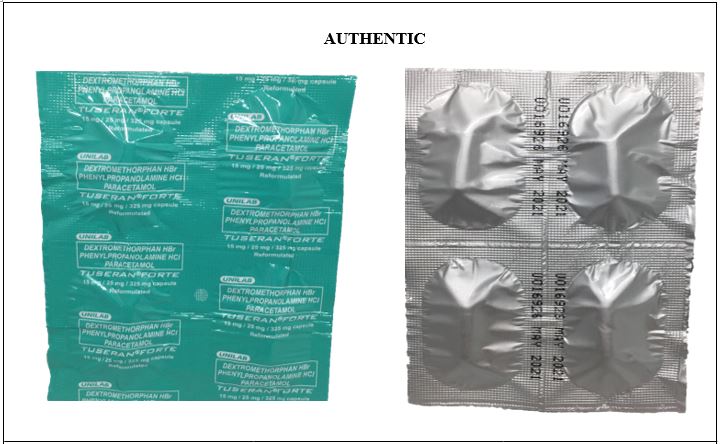

Figure 2. Comparison between the Authentic and Counterfeit Dextromethorphan HBr Phenylpropanolamine HCl / Paracetamol (Tuseran® Forte) 15mg/25mg/325 mg Capsule (Lot Nos. U009818 and U013768)
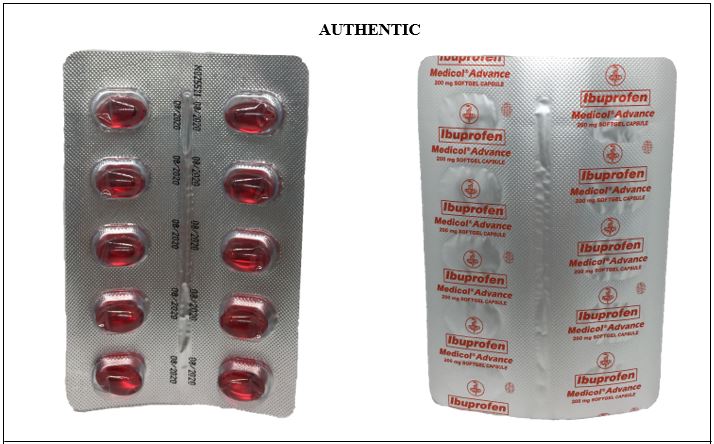
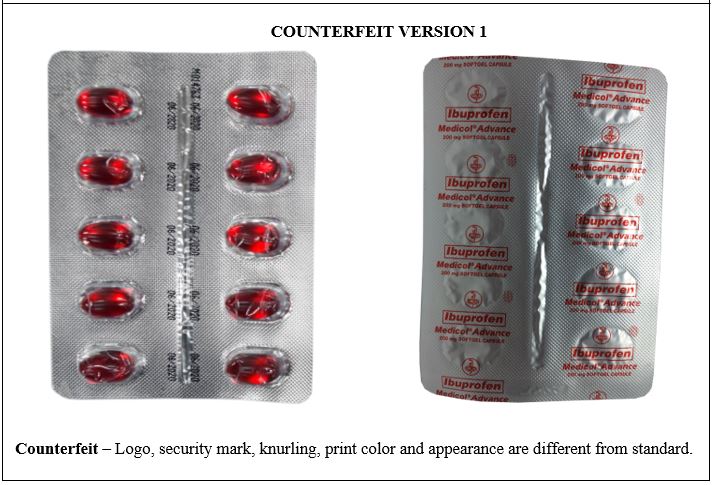 Figure 3-A. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
Figure 3-A. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
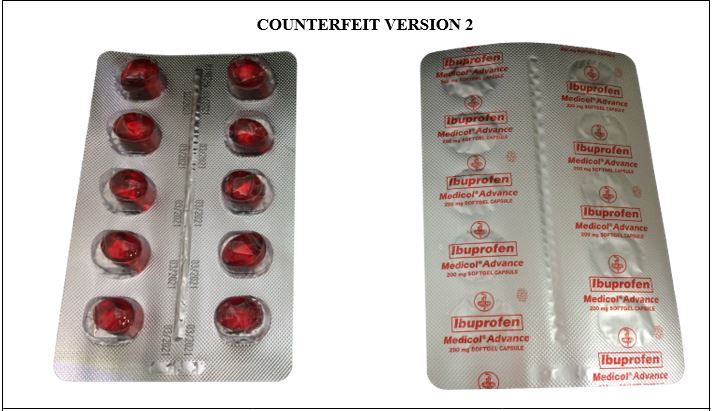
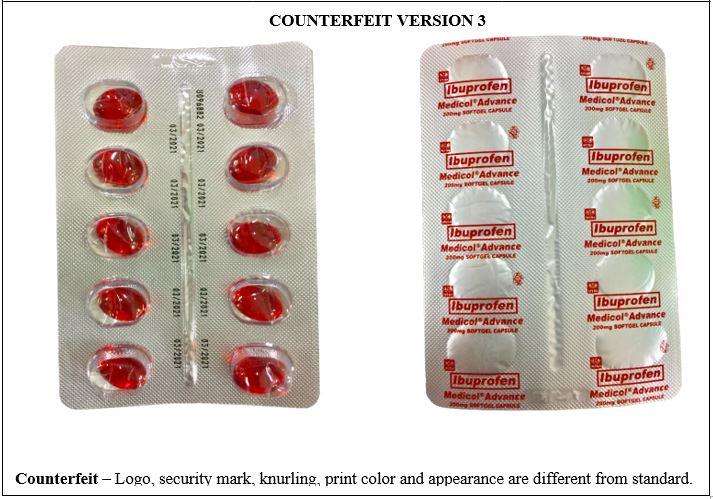
Figure 3-B. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
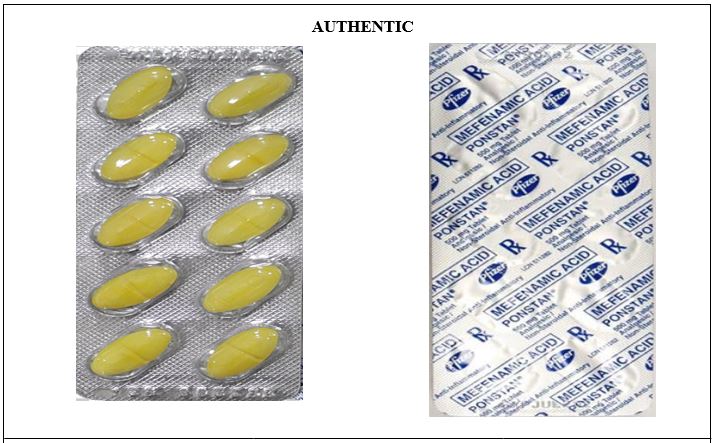
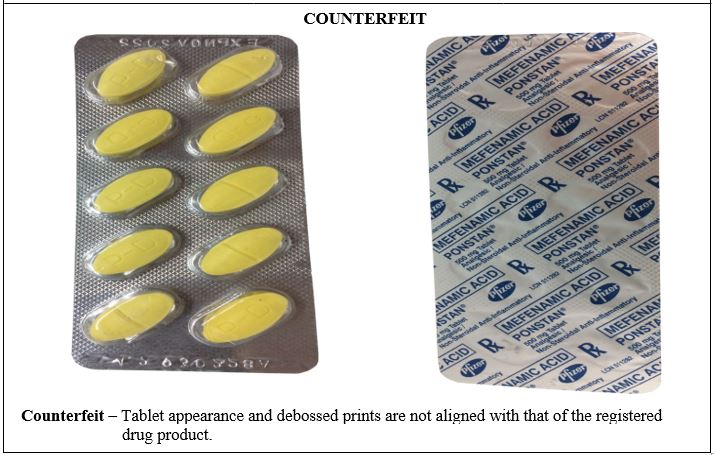
Figure 4. Comparison between the Authentic and Counterfeit Mefenamic Acid (Ponstan®) 500mg Tablet (Lot nos. 930228A and 42930228A)
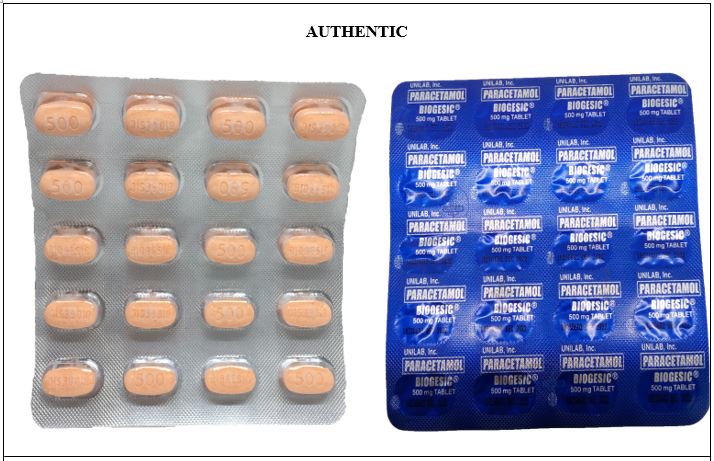
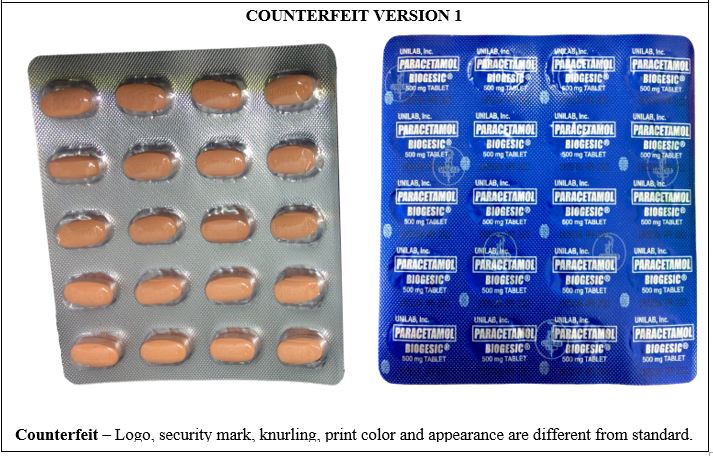
Figure 5-A. Comparison between the Authentic and Counterfeit Paracetamol (Biogesic®) 500mg Tablet (Lot nos. 19047723, 16885778, and 19052306)
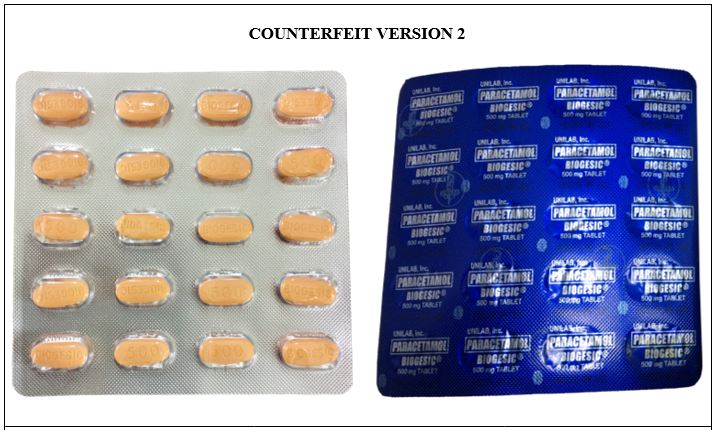
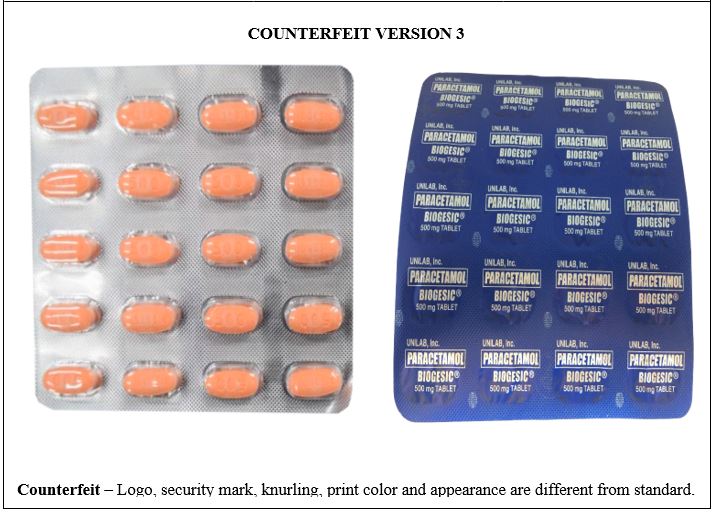
Figure 5-B. Comparison between the Authentic and Counterfeit Paracetamol (Biogesic®) 500mg Tablet (Lot nos. 19047723, 16885778, and 19052306)
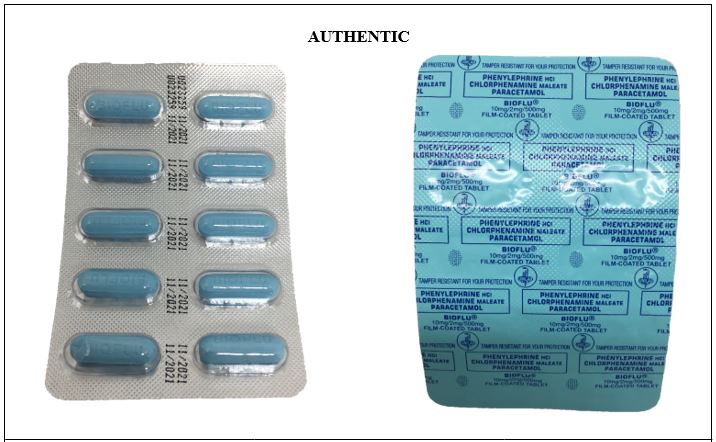
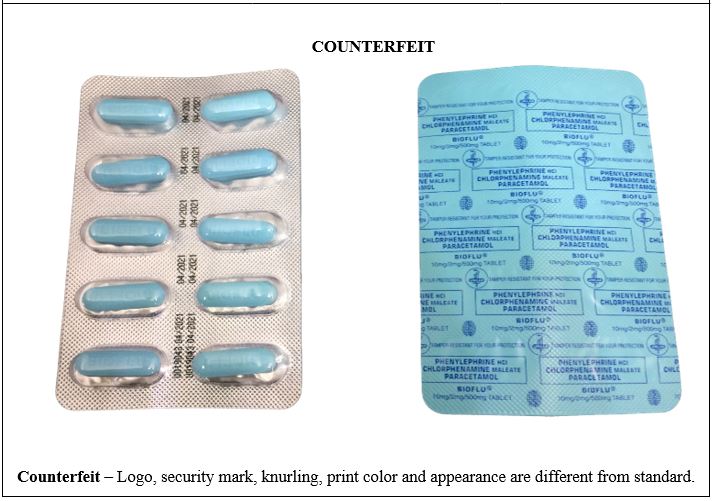
Figure 6. Comparison between the Authentic and Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Bioflu®) 10mg/2mg/500mg Tablet (Lot nos. U019043, 0190415, and 19052306)
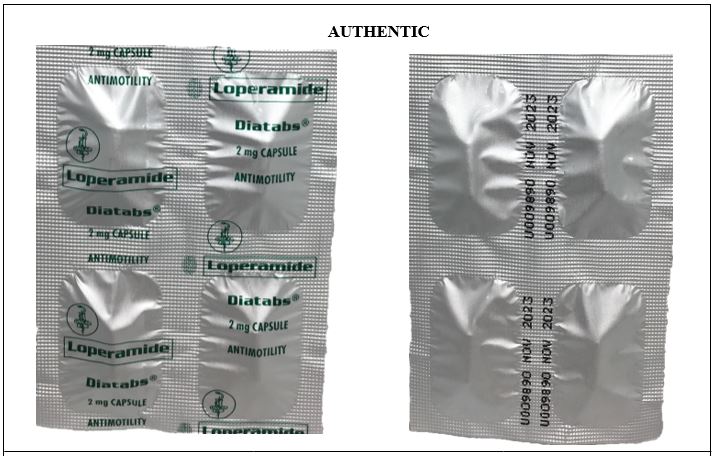
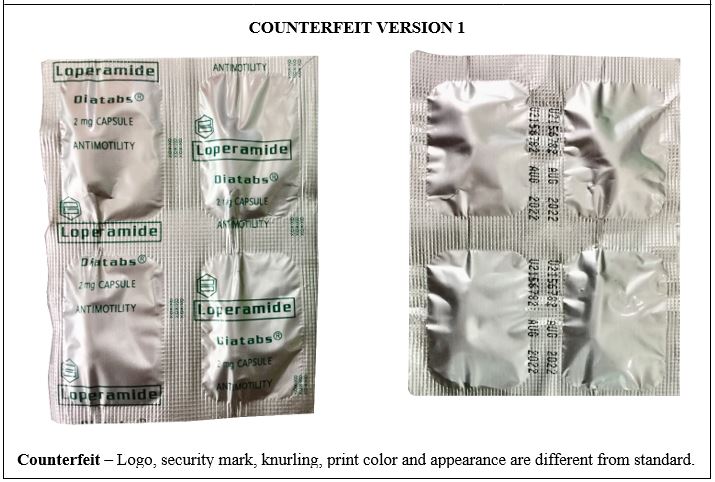
Figure 7-B. Comparison between the Authentic and Counterfeit Loperamide (Diatabs®) 2mg Capsule (Lot nos. U2156782, U455238, and U3167321)
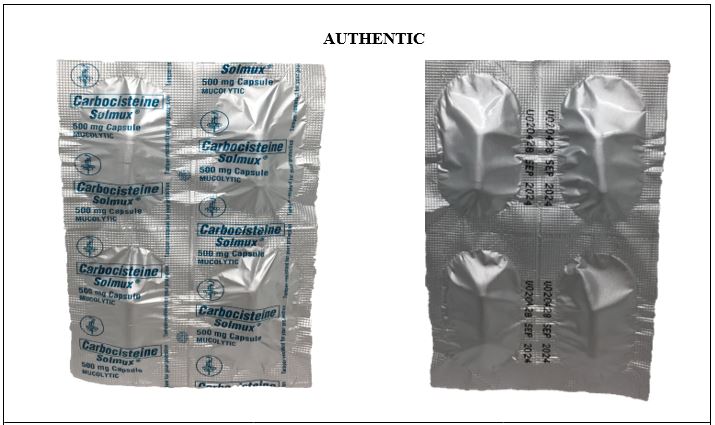
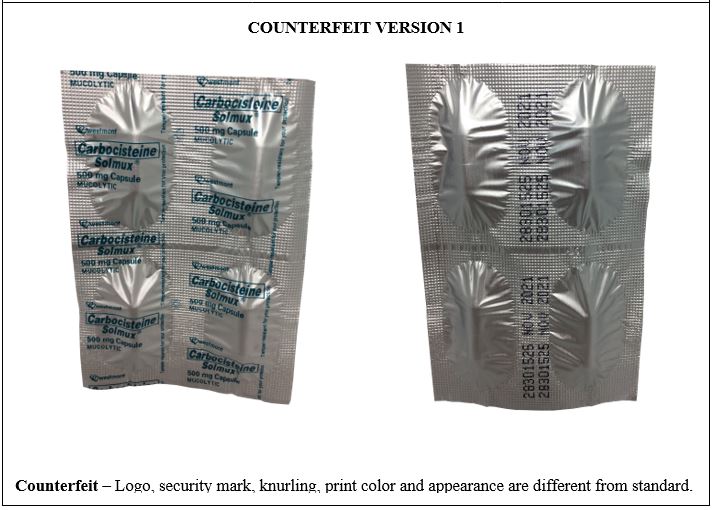
Figure 8-A. Comparison between the Authentic and Counterfeit Carbocisteine (Solmux®) 500mg Capsule (Lot nos. 28301525, U009159, and 28301525)

Figure 8-B. Comparison between the Authentic and Counterfeit Carbocisteine (Solmux®) 500mg Capsule (Lot nos. 28301525, U009159, and 28301525)
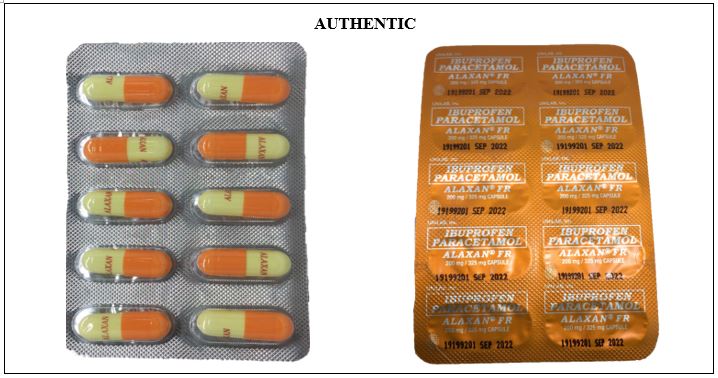
Figure 9-A. Comparison between the Authentic and Counterfeit Ibuprofen / Paracetamol (ALAXAN®)FR 200mg/325mg Capsule (Lot nos. 18090711, 18090705, 19070126, 19030110, 19090828 and 18101901)
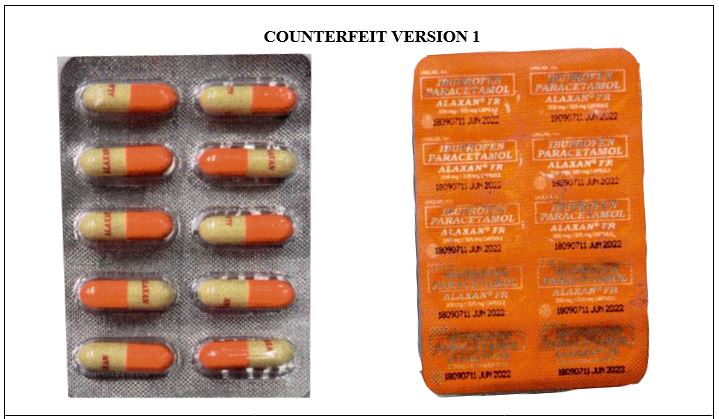
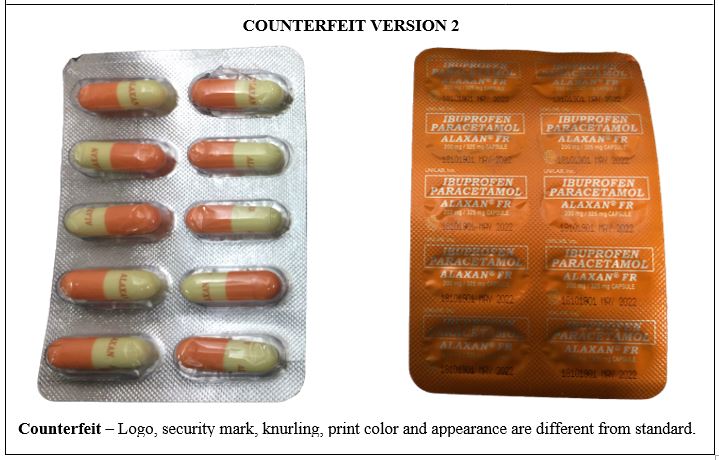
Figure 9-B. Comparison between the Authentic and Counterfeit Ibuprofen / Paracetamol (ALAXAN®) FR 200mg/325mg Capsule (Lot nos. 18090711, 18090705, 19070126, 19030110, 19090828 and 18101901)
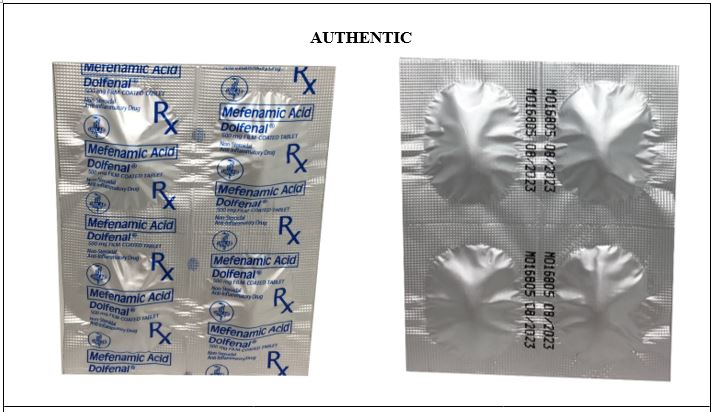
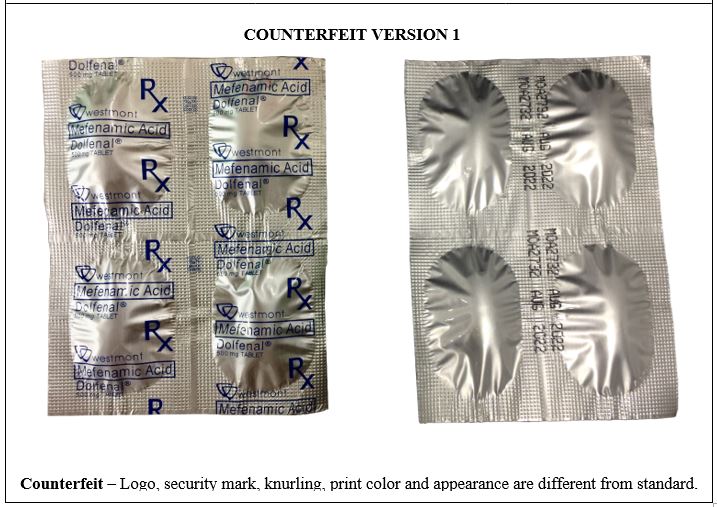
Figure 10-A. Comparison between the Authentic and Counterfeit Mefenamic Acid (Dolfenal®) 500mg Tablet (Lot nos. MOA2732 and MOA1705)
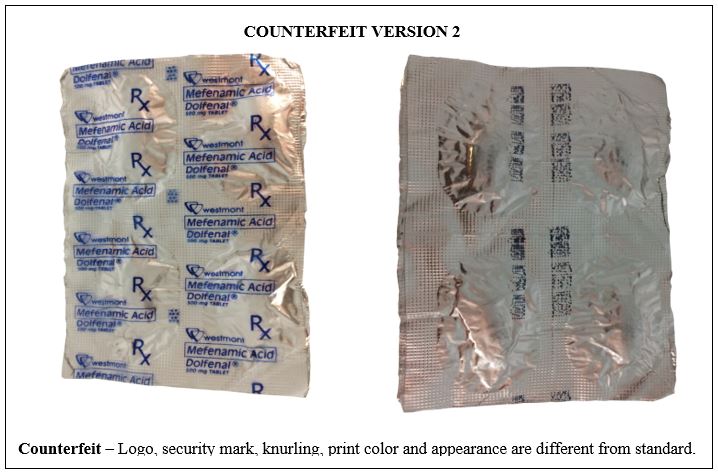
Figure 10-B. Comparison between the Authentic and Counterfeit Mefenamic Acid (Dolfenal®) 500mg Tablet (Lot nos. MOA2732 and MOA1705)
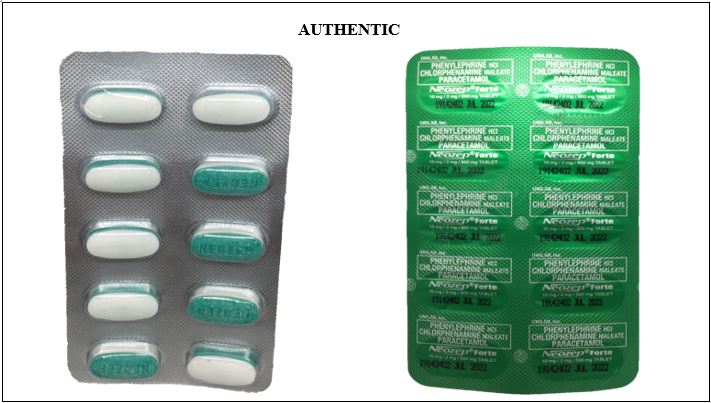
Figure 11-A. Comparison between the Authentic and Verified Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10 mg/2 mg/500 mg Tablet (Lot nos. 19067382, 18212086, and 18012006)
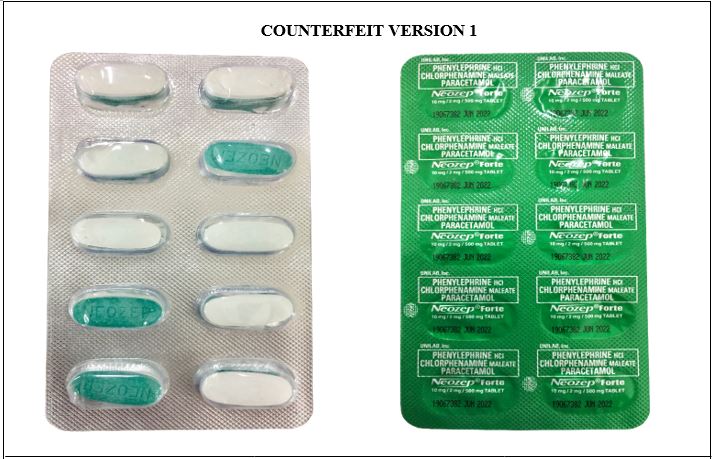
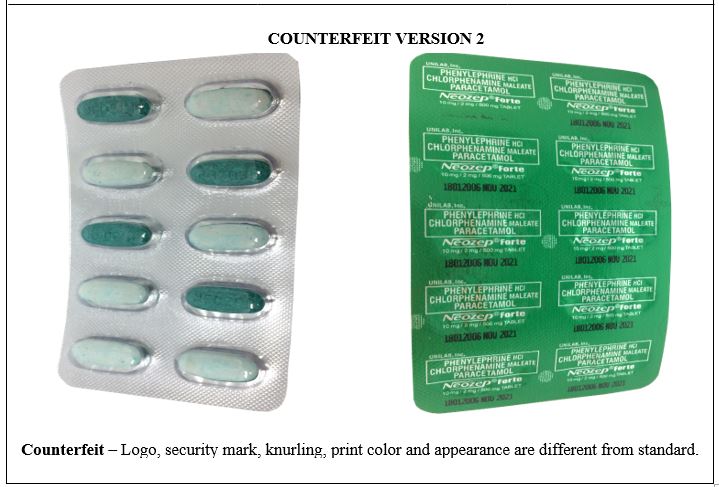
Figure 11-B. Comparison between the Authentic and Verified Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10 mg/2 mg/500 mg Tablet (Lot nos. 19067382, 18212086, and 18012006)
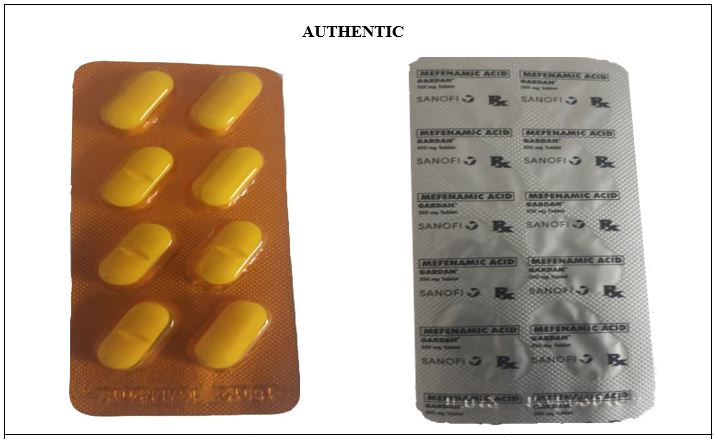
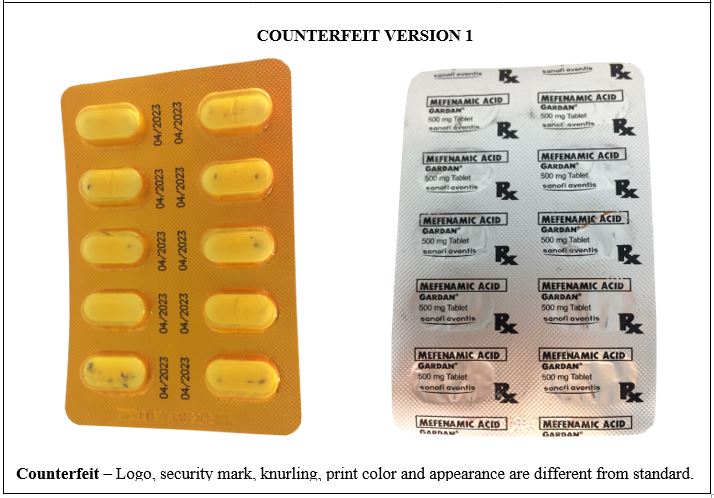
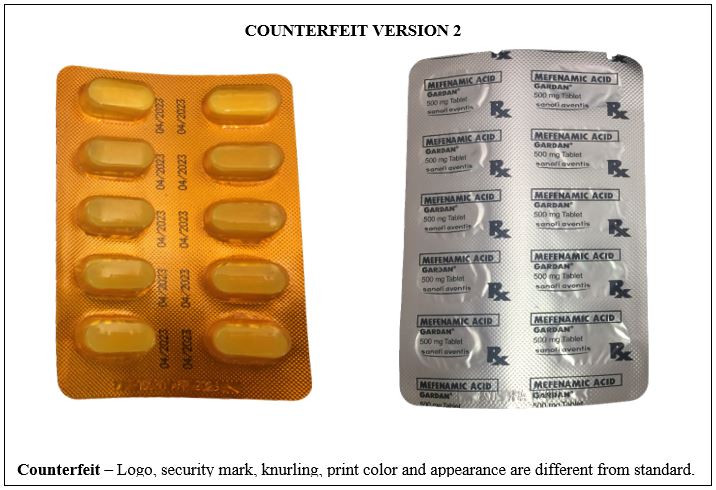
Figure 12-A. Comparison between the Authentic and Counterfeit Mefenamic Acid (Gardan®) 500mg Tablet (Lot nos. 19143 and 19030)
Ang lahat ng healthcare professionals at publiko ay binabalaan tungkol sa paglipana ng mga nasabing pekeng gamot sa merkado na maaaring magdulot ng panganib sa kalusugan ng mga gagamit nito. Ang publiko ay pinapaalalahanan ring bumili lamang sa mga establisyementong lisensyado ng FDA.
Gayundin, ang lahat ng establisyemento ay binabalaang huwag magbenta ng mga pekeng gamot na nagtataglay ng mga nasabing katangian. Ang pagaangkat, pagbebenta at pamamahagi nito ay paglabag sa Republic Act No. 9711 or the Food and Drug Administration Act of 2009, and Republic Act No. 8203 or the Special Law on Counterfeit Drugs. Ang sino mang mapatunayang nagbebenta ng nasabing pekeng produkto ay mapaparusahan.
Hinihiling sa lahat ng Local Government Units (LGUs) and Law Enforcement Agencies (LEAs) na tiyaking ang pekeng produktong ito ay hindi maibebenta o magagamit sa kanilang mga nasasakupan.
Para sa karagdagang impormasyon at katanungan, maaring mag-email sa [email protected]. Upang mag-report ng patuloy na pagtitinda o pangangalakal ng mga pekeng gamut, mag-email sa [email protected], o mag-report gamit ang aming online reporting facility, eReport, sa www.fda.gov.ph/ereport. Maaari ring tumawag sa Center for Drug Regulation and Research sa numerong (02) 8809-5596. Para sa mga hinihinalang hindi kanais-nais na reaksyon sa gamut, i-report agad sa FDA gamit ang link na ito: https://primaryreporting.who-umc.org/Reporting/Reporter?OrganizationID=PH at kumpletuhin ang mga kinakailangang impormasyon.
Ang lahat ay hinihikayat na palaganapin ang mga nakasaad na impormasyon.
Attachment:-> FDA Advisory No.2020-1348-A



