The Food and Drug Administration (FDA) warns all healthcare professionals and establishments on the voluntary recall of affected lots of Covidien DAR™ Airway Products manufactured by Covidien-USA, imported and distributed by Medtronic Philippines, Inc.:
| Item Code | Description | Affected Lot Numbers | |
| 352/5877 | HYGROBAC S ELECST FHME | 18K1188FAX | 18L0219FAX |
| 19B0551FAX | 19C1353FAX | ||
| 20F0524FAX | 20G0358FAX | ||
| 18K1189FAX | 19B0524FAX | ||
| 19E1303FAX | 19E1304FAX | ||
| 18L0558FAX | 19D0943FAX | ||
| 20G0357FAX | |||
| 350/5879 | BARRIERBAC S ELECST FILTER | 18L0217FAX | 18L0218FAX |
| 18L1034FAX | 18L1035FAX | ||
| 18L1265FAX | 19E0227FAX | ||
| 19E0357FAX | 19L0873FAX | ||
| 20D0772FAX | 20D0771FAX | ||
| 20F0456FAX | |||
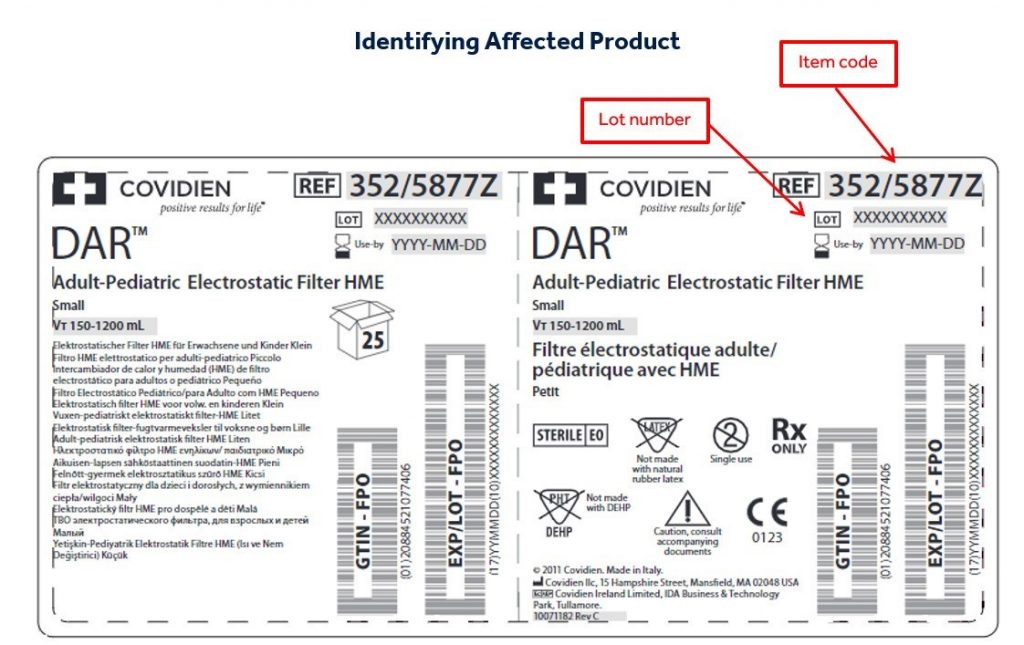
Medtronic International, Ltd. initiated the voluntary recall of the above-mentioned specific lots/batches of Covidien DAR™ Airway Products. Medtronic has concluded its investigation of potential deviations in the ethylene oxide sterilization processes performed by Steril Milano, the former supplier of their sterilization services for the DAR™ Airway Products. Medtronic analyzed the available sterilization data and conducted validation tests on production lots where data was available. The conclusion of their investigation, testing and analysis has resulted in the need to recall certain lots of the DAR™ Airway Products which are currently under quarantine.
The action is being taken because, for certain production lots where no sterilization process data was available, the manufacturer were unable to validate the sterility of these products and are therefore issuing a recall of only these products.
All products subject to this recall are available in two version, either as clean/non-sterile or sterile, except for DAR™ Ty-Care™ closed suction system devices which are available as sterile devices only. Use of DAR™ TyCare™ closed suction system devices where the sterility cannot be assured may be associated with an extremely unlikely risk of infection, particularly given the clinical application of suction devices and when used in accordance with the Instructions for Use.
In light of the foregoing, all concerned healthcare professional, establishments, and the general public are warned to discontinue further use, sale, and distribution of the said affected lots/batches of DAR™ Airway Products.
All FDA Regional Field Offices and Regulatory Enforcement Units, in coordination with law enforcement agencies and Local Government Units, are requested to ensure that product is not sold or made available in the market or areas of jurisdiction.
Any suspected adverse reaction experienced from the use of the medical device but not limited to the lot numbers stated above should be reported immediately to FDA.
For more information and inquiries, kindly contact the FDA Center for Device Regulation, Radiation Health, and Research through e-mail at [email protected], or call (02) 8857-1900 loc. 8301.
Dissemination of this advisory to all concerned is hereby requested.



