The Food and Drug Administration (FDA) warns the public from purchasing and using the following unauthorized toy and childcare article (TCCA) products.
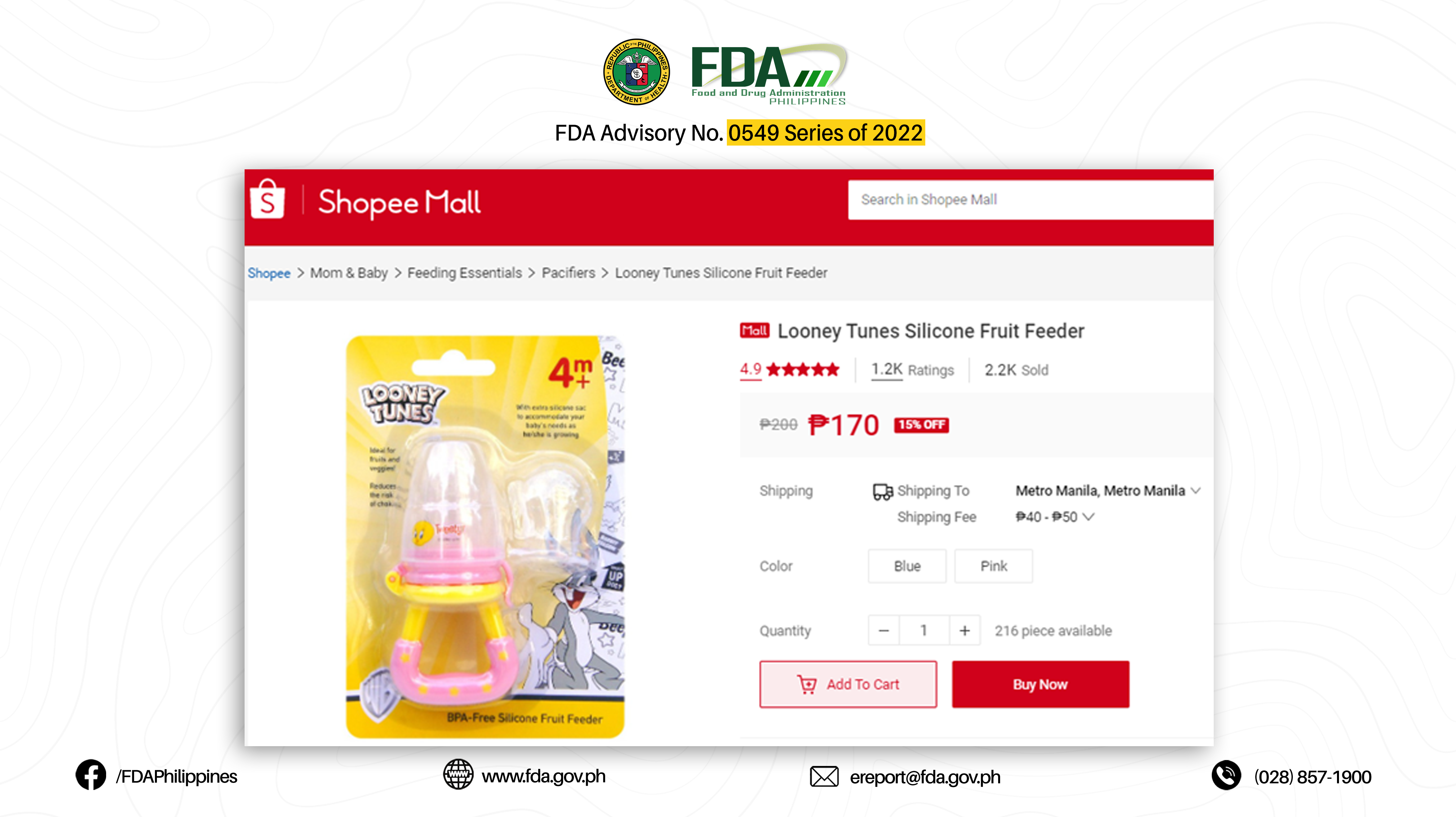
1. LOONEY TUNES BPA-FREE SILICONE FRUIT FEEDER 4M+ (BUGS BUNNY)
2. LOONEY TUNES BPA-FREE SILICONE FRUIT FEEDER 4M+ (TWEETY)
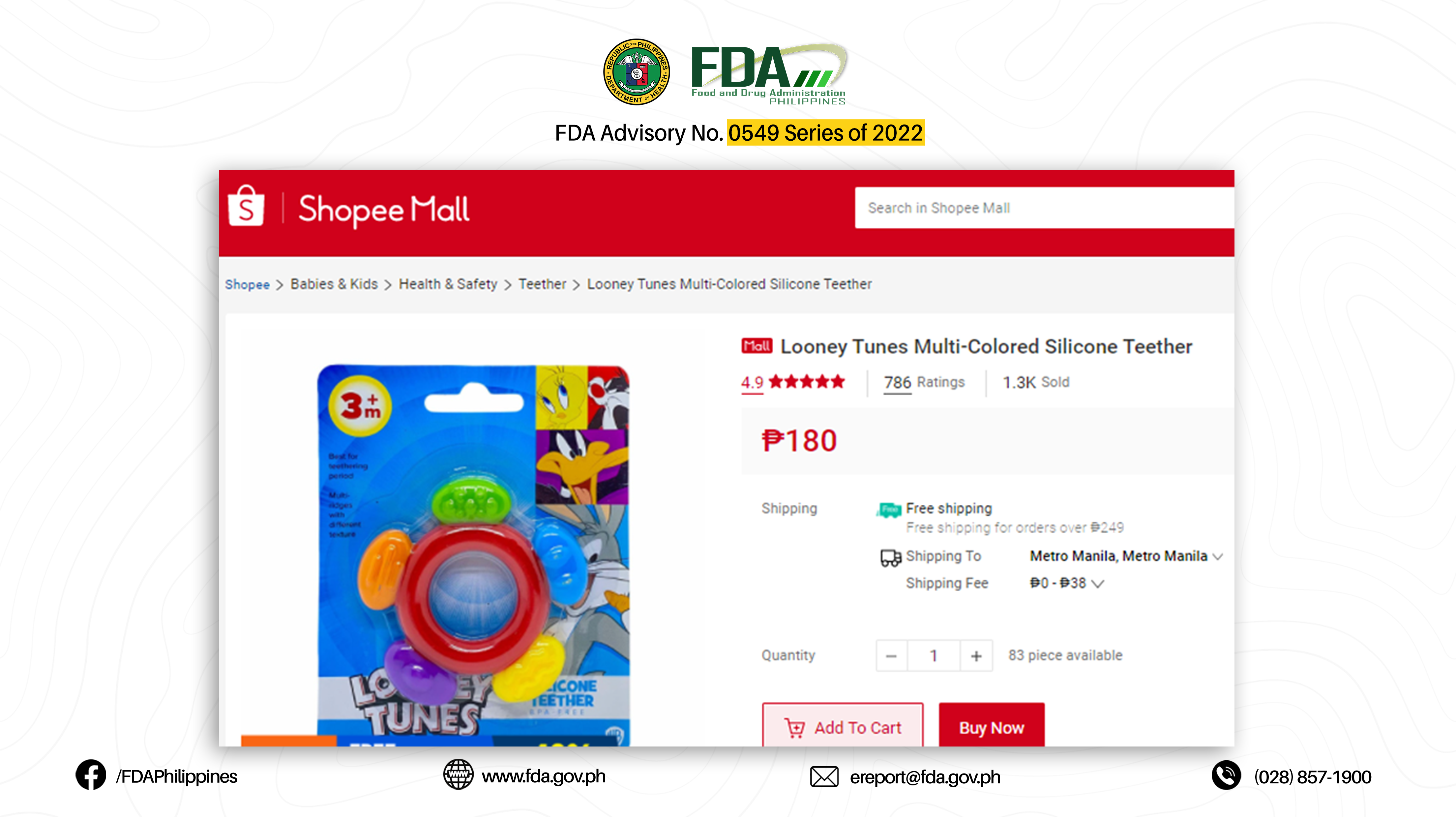
3. LOONEY TUNES BPA-FREE SILICONE TEETHER 3M+
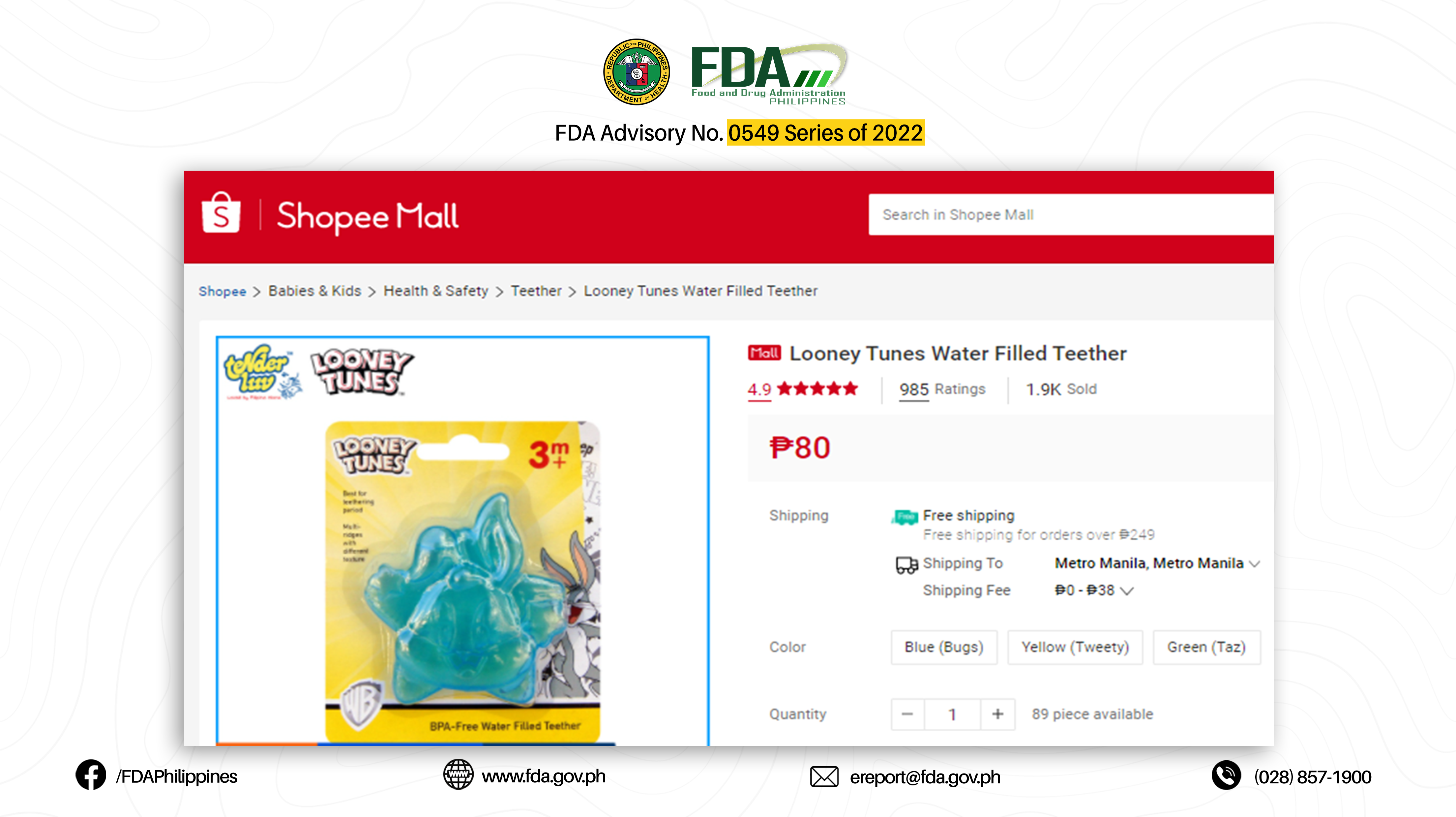
4. LOONEY TUNES BPA-FREE WATER FILLED TEETHER (BLUE BUGS)
5. LOONEY TUNES BPA-FREE WATER FILLED TEETHER (YELLOW TWEETY)
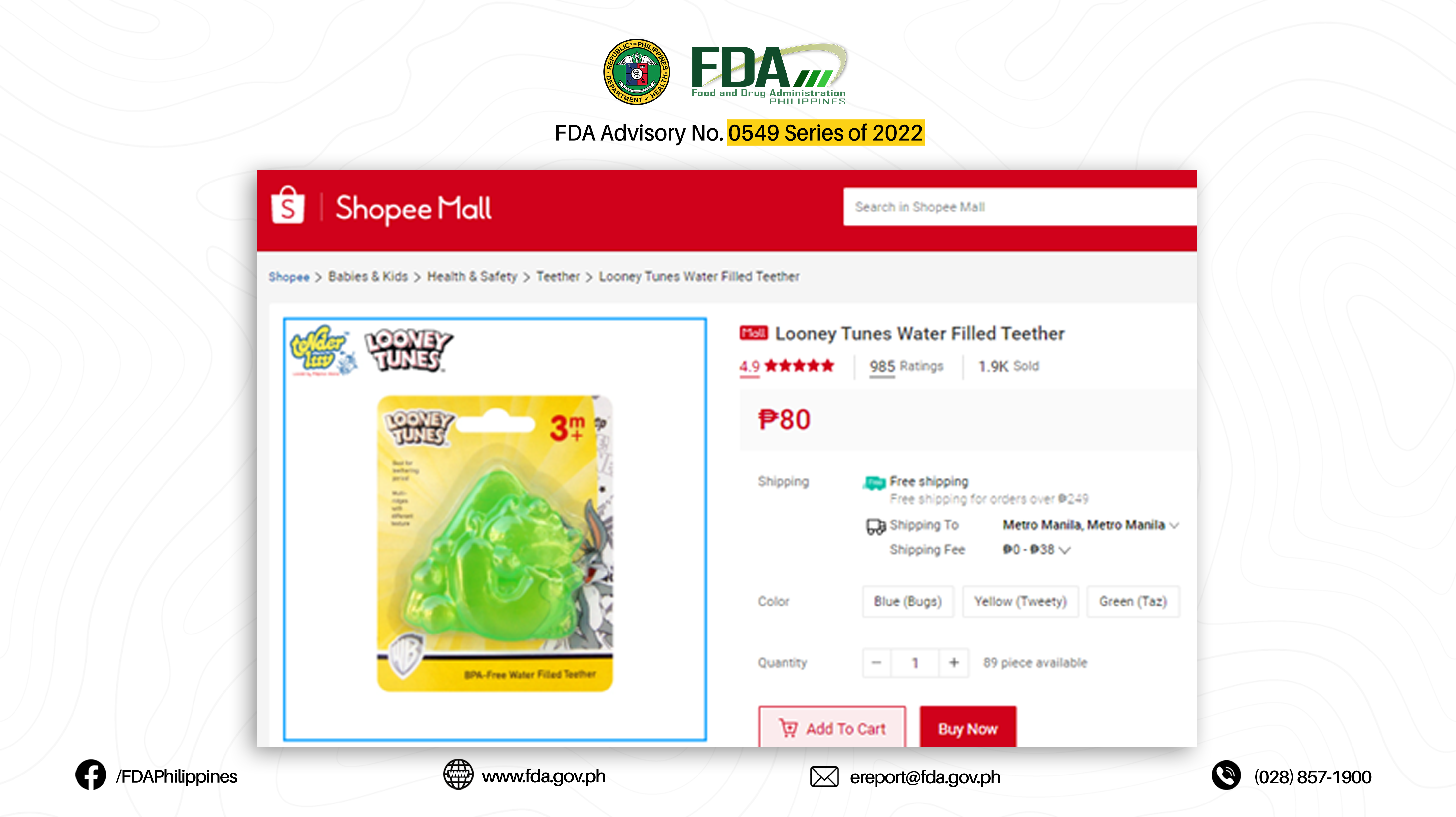
6. LOONEY TUNES BPA-FREE WATER FILLED TEETHER (GREEN TAZ)
The abovementioned products were verified by FDA through postmarketing surveillance and show no valid Certificate of Product Notification (CPN) as of 07 March 2022. Pursuant to Book II, Article I, Section 1 (a) of the Rules and Regulations Implementing Republic Act No. 9711, otherwise known as the “Food and Drug Administration Act of 2009”, the manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising, or sponsorship of any health product without the proper authorization from the FDA is prohibited.
Since the abovementioned unauthorized toy and childcare article product has not gone through the notification process of the FDA, the agency cannot assure their quality and safety. The use of such violative product may pose health risks to consumers.
Potential hazards may come from ingredients that are not allowed to be part of a toy and childcare article. The use of substandard and possibly adulterated toy and childcare article product may result to health risks including, but not limited to, endocrine disruption and reproductive or development effects; or may result to injury, choking or suffocation due to its small or broken parts.
In light of the foregoing, the public is advised not to purchase the aforementioned violative cosmetic product. Always check if a product is notified with the FDA by using the FDA Verification Portal feature accessible at https://verification.fda.gov.ph which may be used by typing in the name of the product before the purchase and/or using the cosmetic products.
All concerned establishments are warned not to distribute violative cosmetic product until they have fully complied with the rules and regulation of the FDA.
All FDA Regional Field Offices and Regulatory Enforcement Units, in coordination with law enforcement agencies and Local Government Units, are requested to ensure that violative products are not sold or made available in the market or areas of their jurisdiction.
To report any sale, distribution, complaint and/or adverse event on the use of the violative cosmetic products, the online reporting facility, eReport can be accessed at [email protected], or call us at the Center for Cosmetics and Household/Urban Hazardous Substances Regulation and Research (CCHUHSRR) hotline (02) 8857-1900 loc. 8113 or 8107.
Dissemination of this advisory to all concerned is hereby requested.