The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device products:
1. CYTOCARE 532 5 ML – 0.17 FL. OZ.
2. CYTOCARE C LINE 640 MB REVITALISATION SOLUTION – HYALURONIC ACID
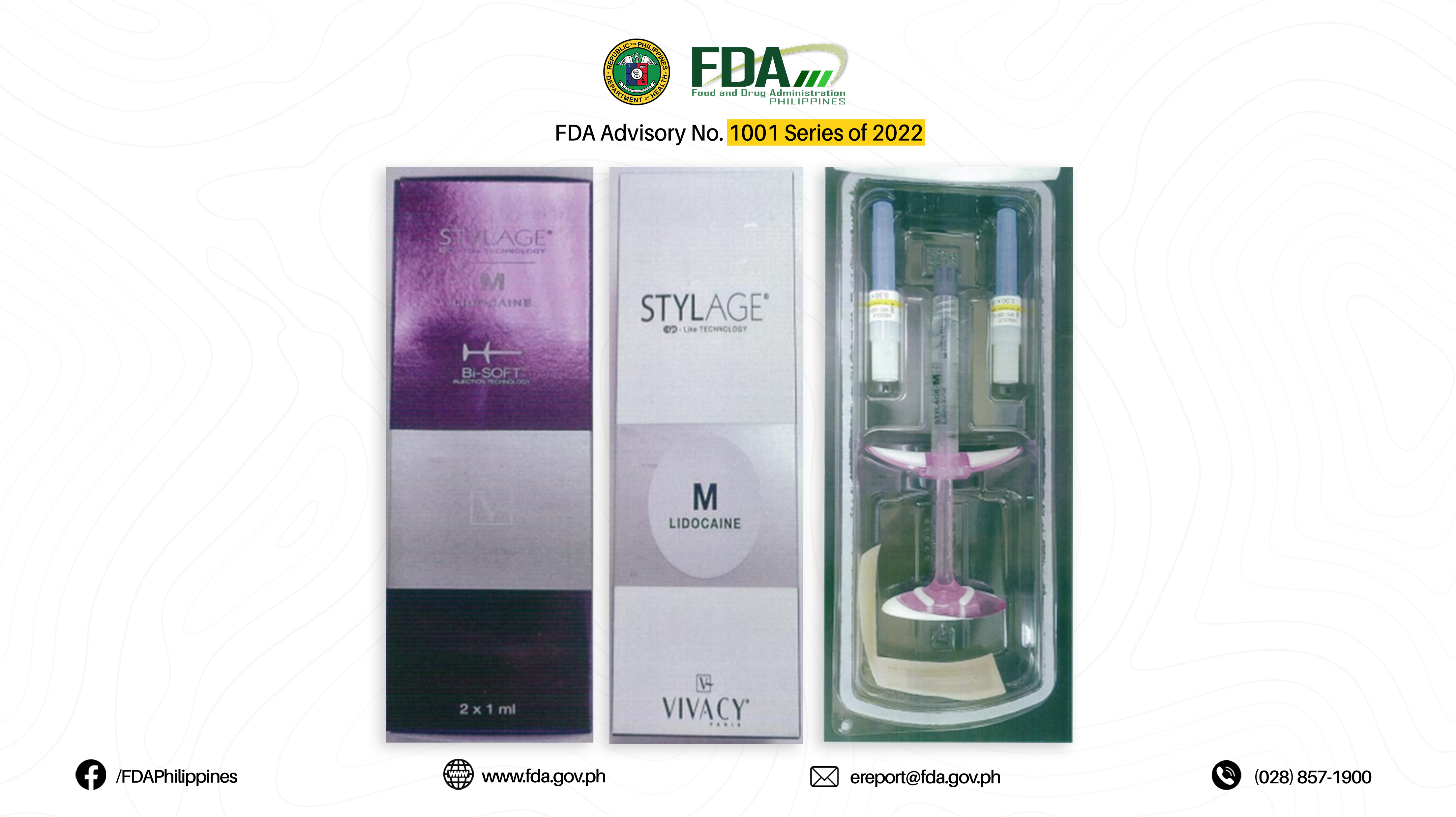
3. JUVEDERM® ULTRA 3 2X1 ML, STYLAGE IPN – LIKE TECHNOLOGY M LIDOCAINE
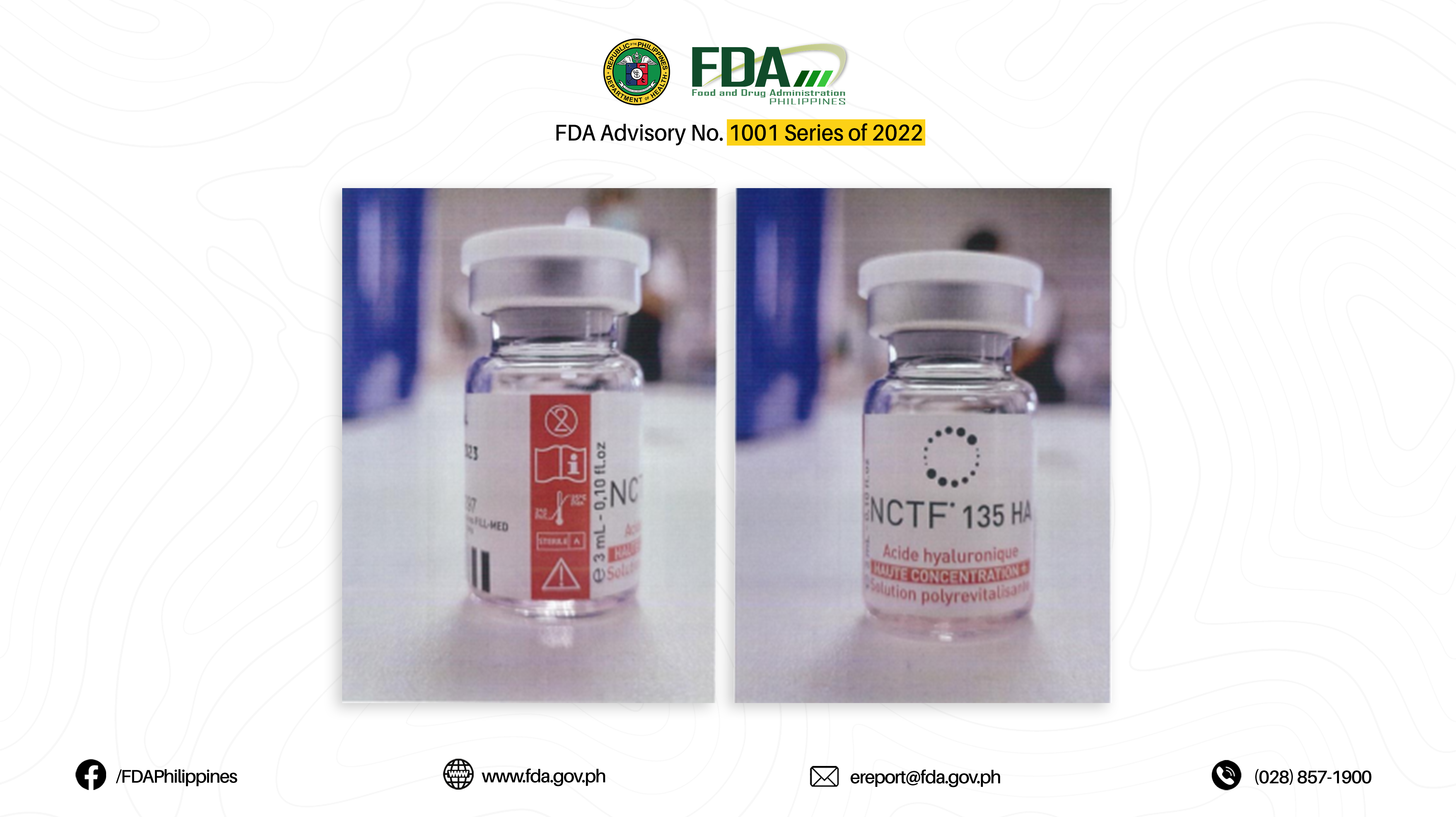
4. ACID HYALURONIQUE, HAUTE CONCENTRATION + SOLUTION POLYREVITALISANTE (NCTF 135 HA)
5. STYLAGE IPN – LIKE TECHNOLOGY M LIDOCAINE
6. REJURAN® HEALER TRUESKIN ESSENCE 2ML X 2EA
The FDA verified through post-marketing surveillance that the above mentioned medical device products are not registered and no corresponding Product Registration Certificates have been issued. Pursuant to the Republic Act No. 9711, otherwise known as the “Food and Drug Administration Act of 2009”, the manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising or sponsorship of health products without the proper authorization is prohibited.
Since these unregistered medical device products have not gone through evaluation process of the FDA, the agency cannot assure its quality and safety.
All concerned establishments are warned not to distribute, advertise, or sell the said violative medical device products until the Product Registration Certificates are issued, otherwise, regulatory actions and sanctions shall be strictly pursued. Always check if a product has been registered with the FDA before purchasing it by making use of the embedded Search feature of the FDA website accessible at www.fda.gov.ph. You may also look for the FDA Registration number on the product label in the form of either CMDR-xxx, DVR-xxx, or MDR-xxx.
All Law Enforcement Agencies (LEAs) and Local Government Units (LGUs) are requested to ensure these products are not sold or made available in the market or areas of jurisdiction.
The Bureau of Customs is urged to restrain the entry these unregistered products.
For more information and inquiries about this advisory, kindly contact the FDA CDRRHR through e-mail at [email protected] indicating on the subject the concerned Advisory, or call (02) 8857-1900 loc. 8301.