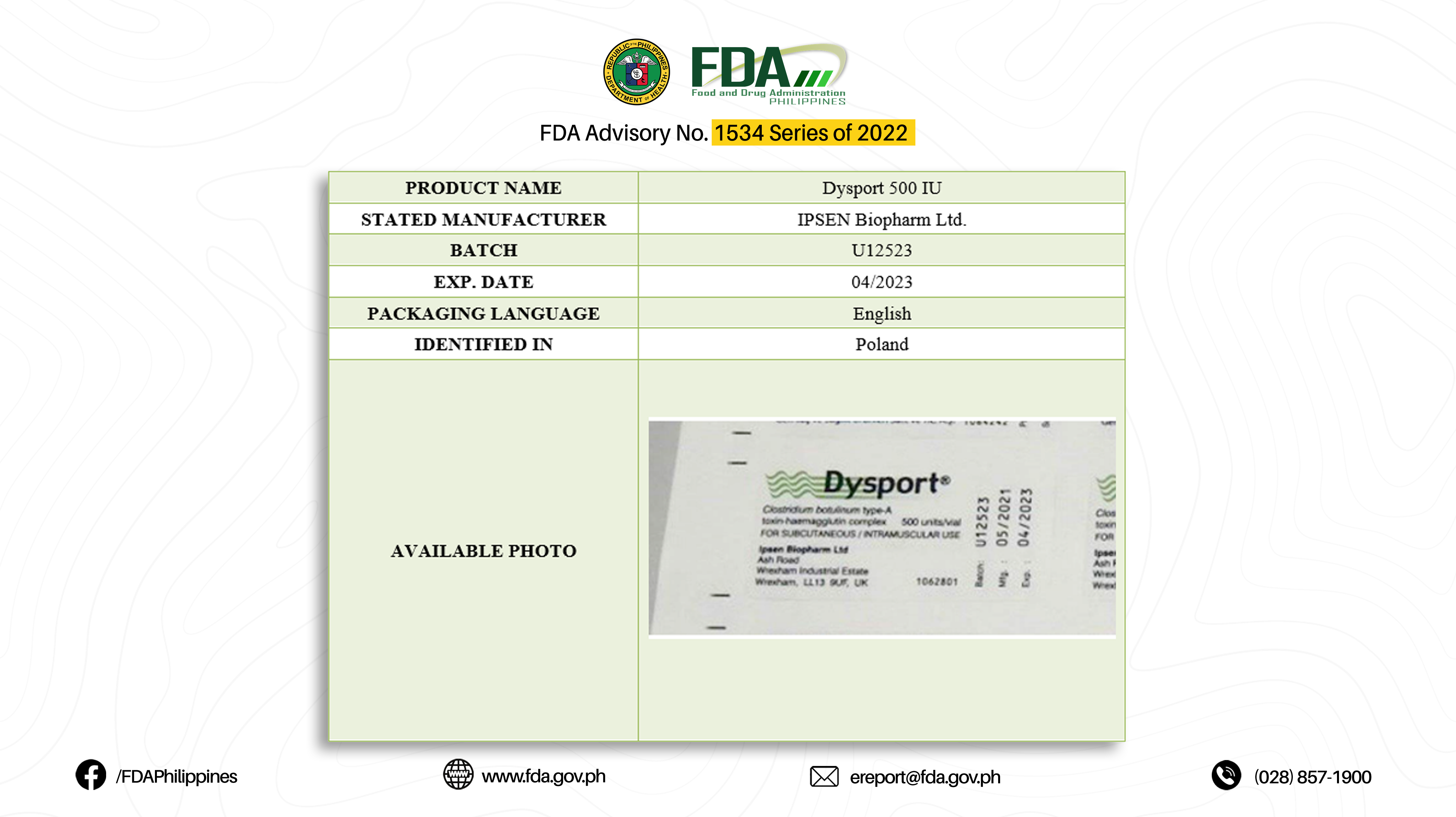
The Food and Drug Administration (FDA) notifies the public on the WHO Medical Product Alert on five (5) falsified Clostridium Botulinum Toxin Type A with brand name “Dysport” which were detected in Europe and Eastern Mediterranean regions from May to July 2022:
The FDA strongly advises the public to be vigilant on the circulation of this falsified drug product since this poses a particular risk to patients as they are administered subcutaneously or intramuscularly, and their sterility, quality, and safety are unknown. A falsified drug product deliberately or fraudulently misrepresents its identity, composition, or source and its safety and quality are unknown. The genuine manufacturer, IPSEN Biopharm Ltd., confirmed that all the products and their variable data (batch numbers, manufacturing, and expiry dates) referenced are falsified, including discrepancies in the packaging languages, type of vial, and, printing errors on the cartons.
This is to emphasize that the authentic Botulinum Toxin Type A 500 Units Lyophilized Powder for Injection (IM/SC) [Dysport] is registered with FDA with Registration No. BR-540 and used to treat symptoms of cervical dystonia, glabellar lines (wrinkles), and spasticity.
Therefore, all Local Government Units (LGU) and Law Enforcement Agencies (LEAs), after the issuance of this advisory, are requested to ensure that this falsified drug product is not sold or not administered to patients in their localities or areas of jurisdiction.
For more information and inquiries, please e-mail us at [email protected]. To report unauthorized sale or distribution of the abovementioned, kindly e-mail us via [email protected]. You may also call the Center for Drug Regulation and Research at telephone number (02) 8809-5596.
Dissemination of the information to all concerned is highly requested