The Food and Drug Administration (FDA) notifies the public on the WHO Medical Product Alert on eight (8) substandard (contaminated) pediatric drug products that have been identified in Indonesia in October 2022:
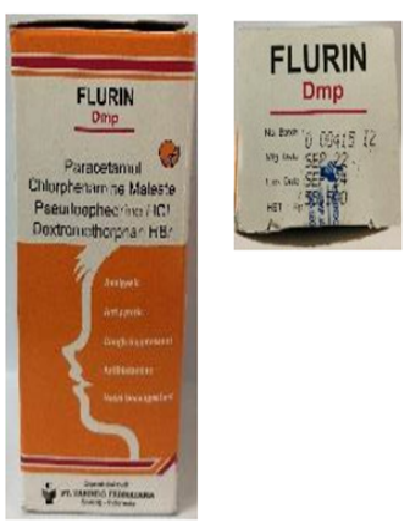
Figure 1. Termorex syrup, Flurin DMP syrup, and Unibebi Cough Syrup detected in Indonesia
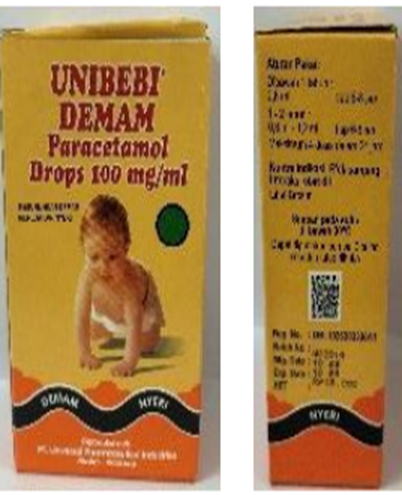
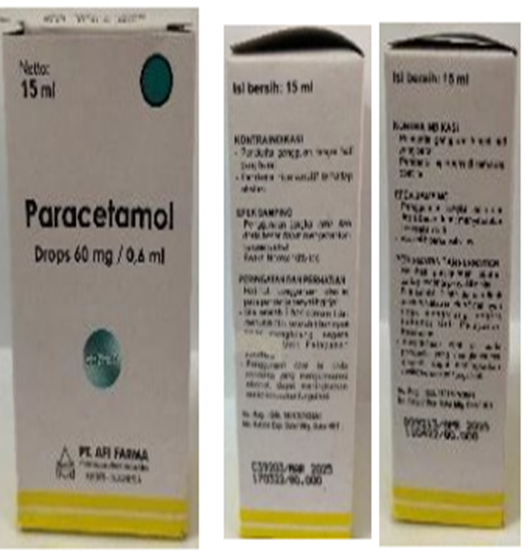
Figure 2. Unibebi Demam Paracetamol Syrup, Unibebi Fever Drops, and Paracetamol Drops detected in Indonesia
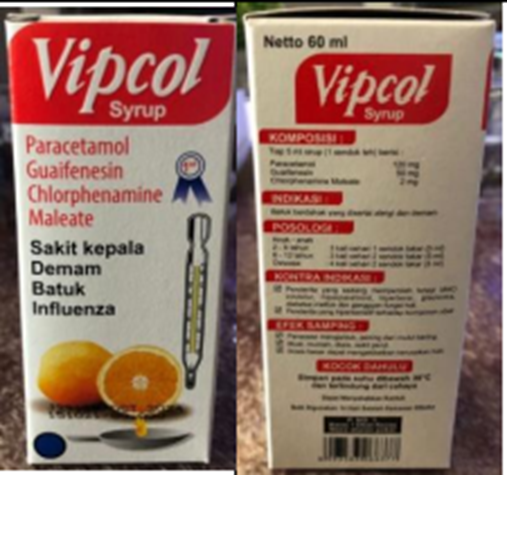
Figure 3. Paracetamol syrup (mint) and Vipcol syrup detected in Indonesia
The FDA strongly advises the public to be vigilant on the circulation of these substandard drug products since its contaminants, Diethylene Glycol and Ethylene Glycol, are toxic to humans when consumed above the acceptable limit and may result to abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death. Substandard drug products are products that fail to meet either their quality standards or specifications. To date, the Badan POM Indonesia ordered the concerned pharmaceutical industries to withdraw the drug syrups from circulation throughout Indonesia and destroy all the affected batches.
This is to emphasize that the abovementioned drug products are not registered with FDA. However, it is important to detect and remove these products from circulation to prevent harm to patients.
Therefore, all Local Government Units (LGU) and Law Enforcement Agencies (LEAs), after the issuance of this advisory, are requested to ensure that these substandard drug products are not sold or not administered to patients in their localities or areas of jurisdiction. Furthermore, manufacturers of liquid dosage forms, especially syrups that contain excipients such as propylene glycol, polyethylene glycol, sorbitol, and/or glycerin/glycerol, are urged to test for the presence of the stated contaminants before use in production of pharmaceutical products.
For more information and inquiries, please e-mail us at [email protected]. To report unauthorized sale, or distribution of the abovementioned, kindly e-mail us via [email protected]. You may also call the Center for Drug Regulation and Research at telephone number (02) 8809-5596.
Dissemination of the information to all concerned is highly requested.