The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device products:
1. “A2-DX (FOR PREVENTING INVERSION/EVERSION WITH ANTERIOR STABILITY)”
2. “BODYMATE ELBOW (LIGHT COMPRESSION AND PROTECTION)”
3. “BODYMATE ELBOW (LIGHT COMPRESSION AND PROTECTION)”
4. “FILMISTA ANKLE (LIGHT COMPRESSION & PROTECTION OF THE ANKLE)”
5. “ZK-1 (SLEEVE-TYPE KNEE SUPPORT WITH PROTECTION PAD)”
6. “A1 (FOR PREVENTING INVERSION)”
7. “ZK-7 (FOR SPRAINS OF THE ACL, MCL, & LCL)”
8. “RK-2 (FOR TROUBLES AROUND PATELLA CAUSED BY RUNNING)”
9. “FINGER WRAP DOUBLE (SUPPORT FOR SPRAINED FINGERS)”
10. “FINGER WRAP SINGLE (FOR SPRAINED FINGER)”
11. “ARM SLEEVES (COMPRESSION AND SUPPORT)”
12. “BODYMATE KNEE (LIGHT COMPRESSION AND PROTECTION)”
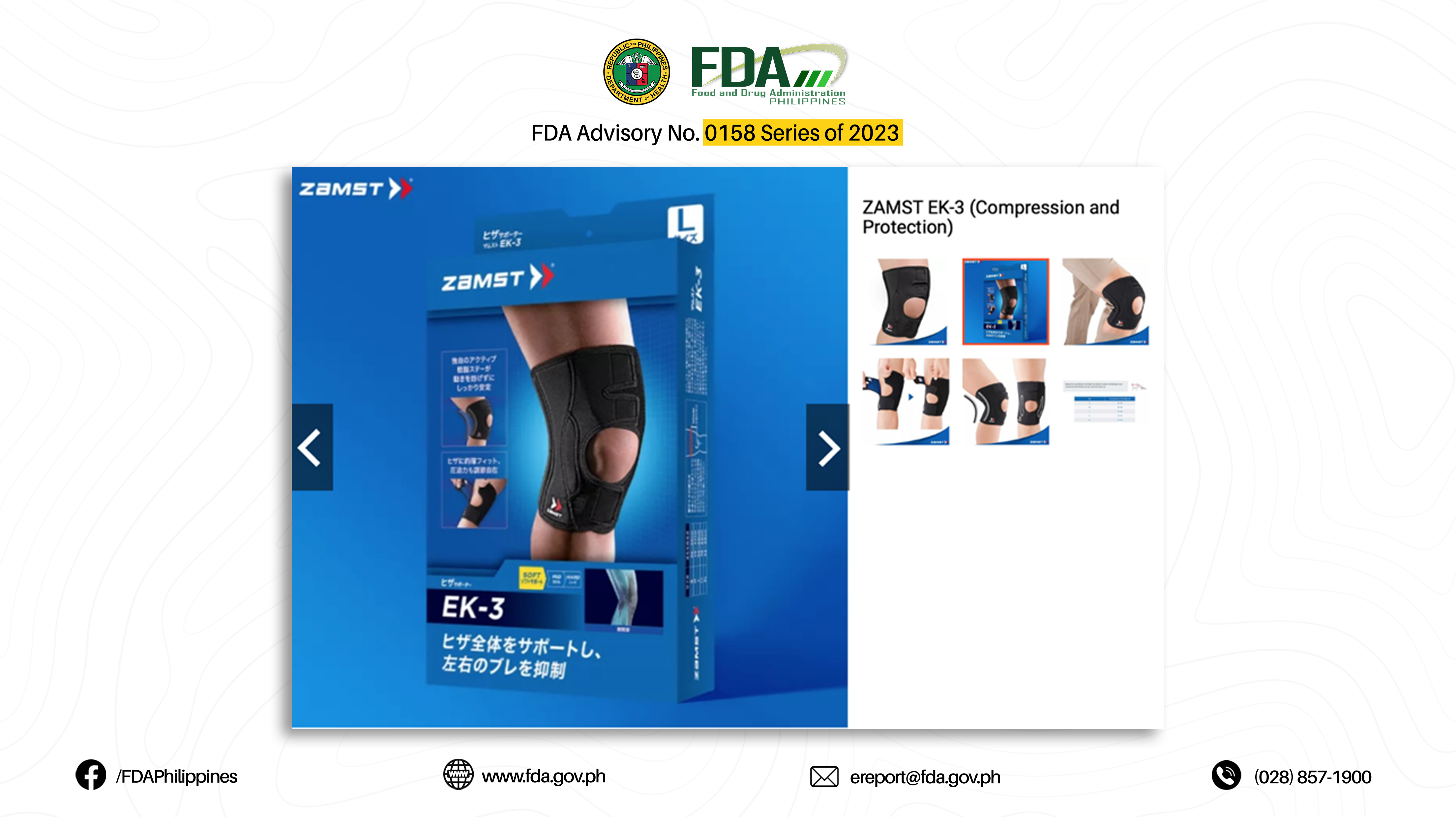
13. “EK-3 (COMPRESSION AND PROTECTION)”
14. “ZW-5 (MODERATE BACK SUPPORT WITH 3D BACK PANEL)”
15. “THUMB GUARD (WITH CUSTOM FIT)”
16. “IW-2 SET (FOR SHOULDER AND BACK)”
17. “FOOTCRAFT AGILITY GRIP (FUNCTIONAL INSOLES)”
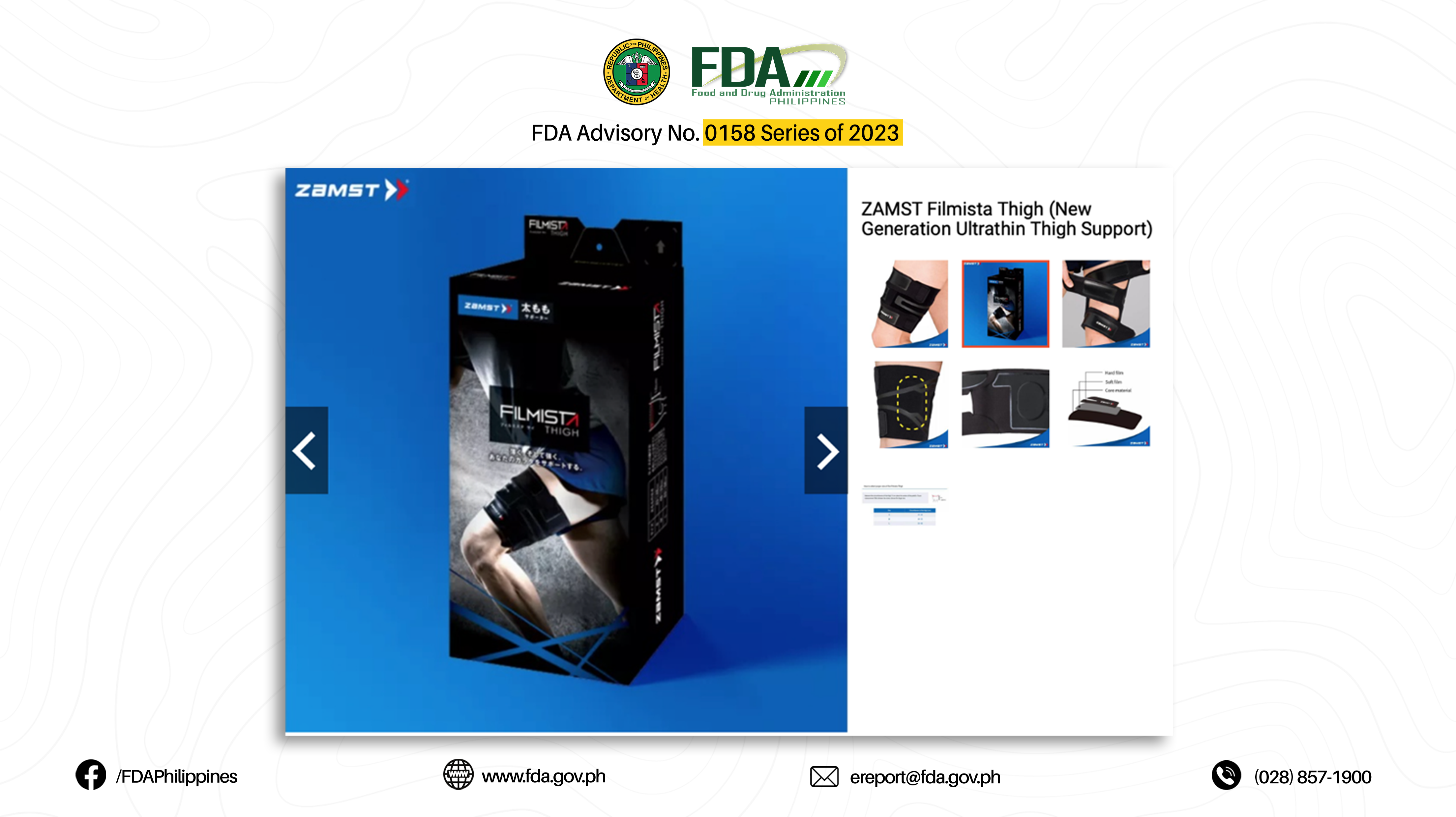
18. “FILMISTA THIGH (NEW GENERATION ULTRATHIN THIGH SUPPORT)”
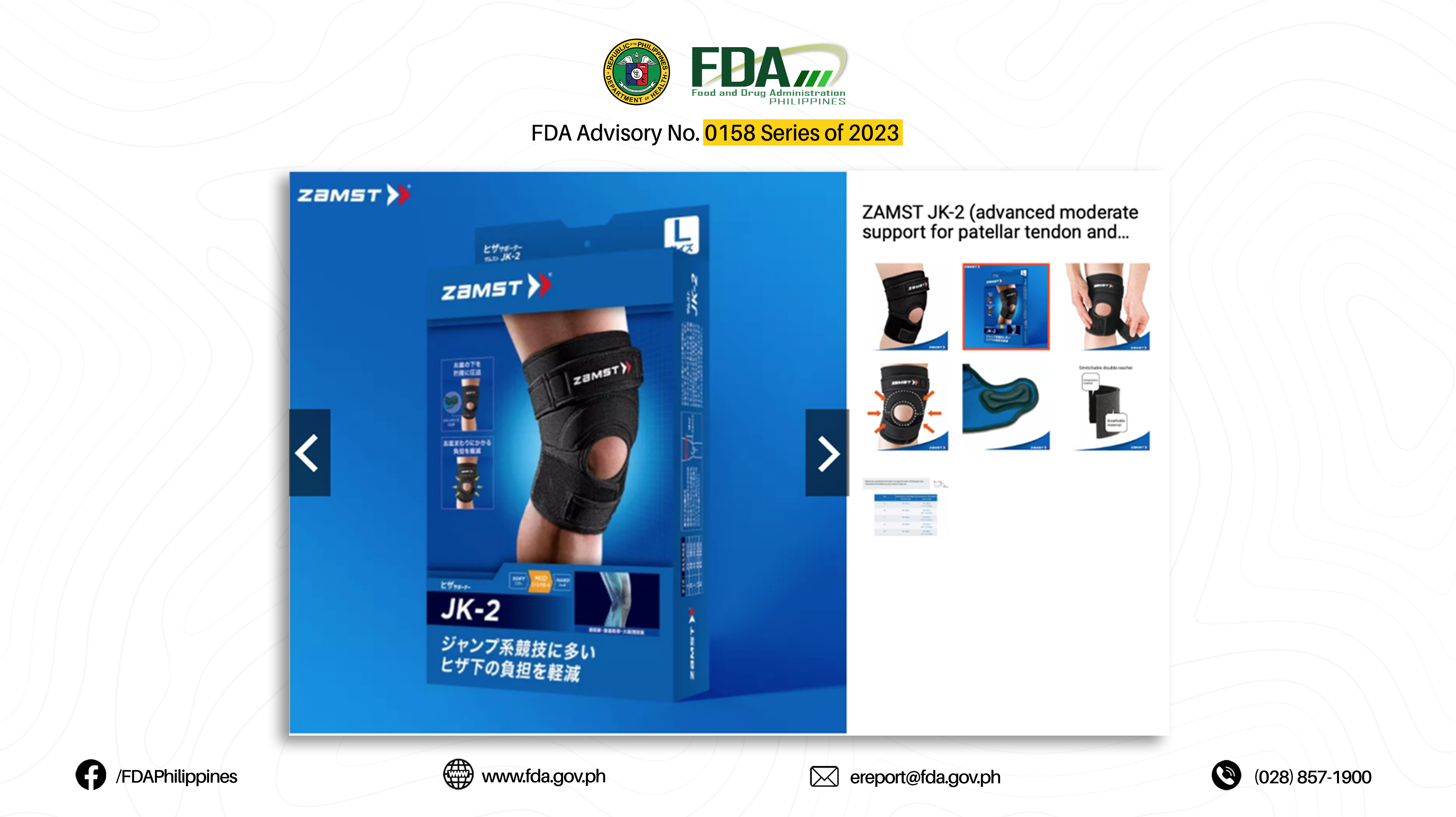
19. “JK-2 (ADVANCED MODERATE SUPPORT FOR PATELLAR TENDON AND PATELLA)”
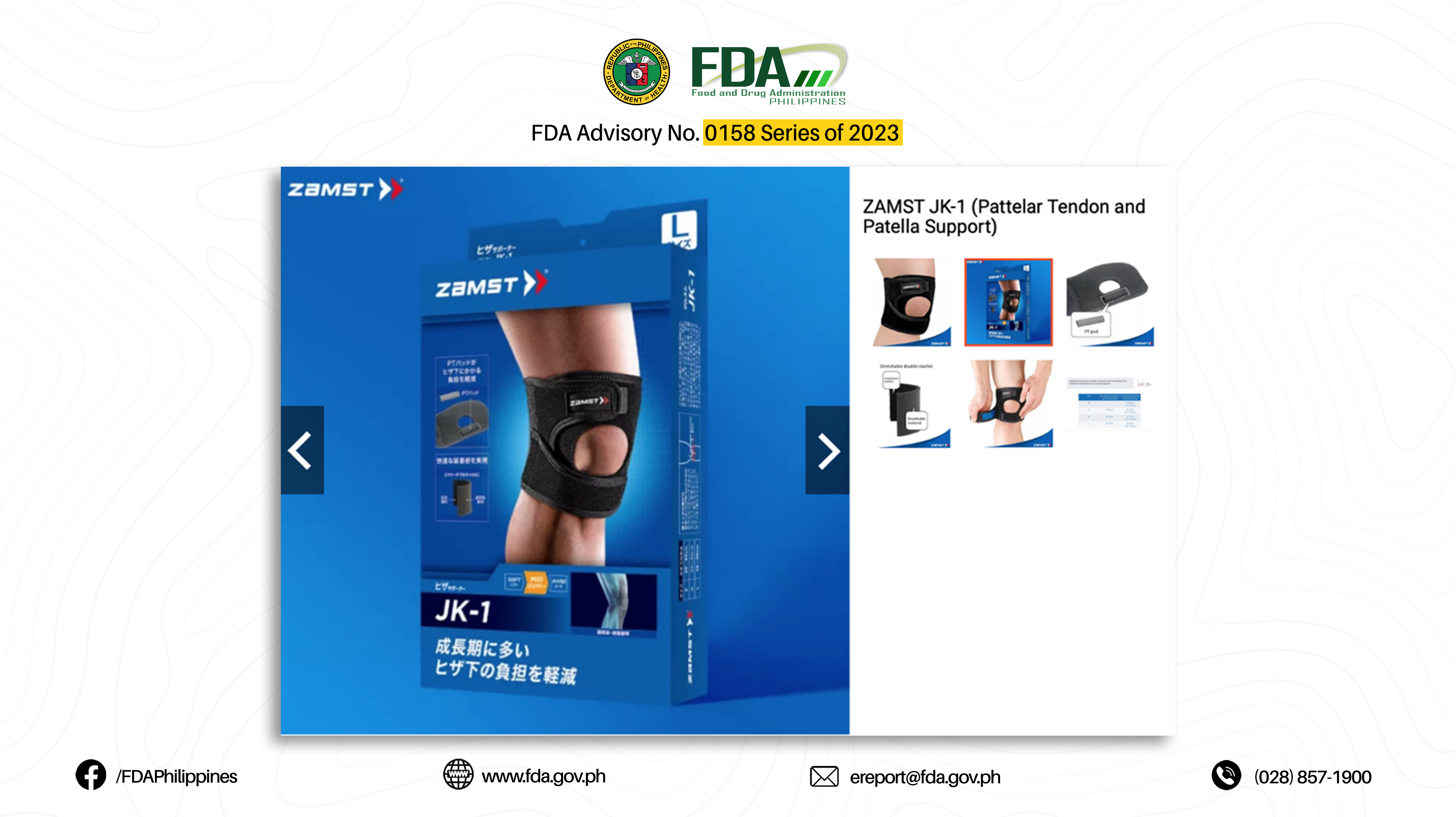
20. “JK-1 (PATTELAR TENDON AND PATELLA SUPPORT)”
21. “FOOTCRAFT STANDARD (FUNCTIONAL INSOLES)”
22. “BODYMATE WRIST (LIGHT COMPRESSION AND PROTECTION)”
23. “FOOTCRAFT CUSHIONED FOR SPIKE (FUNCTIONAL INSOLES)”
24. “THUMB GUARD SOFT”
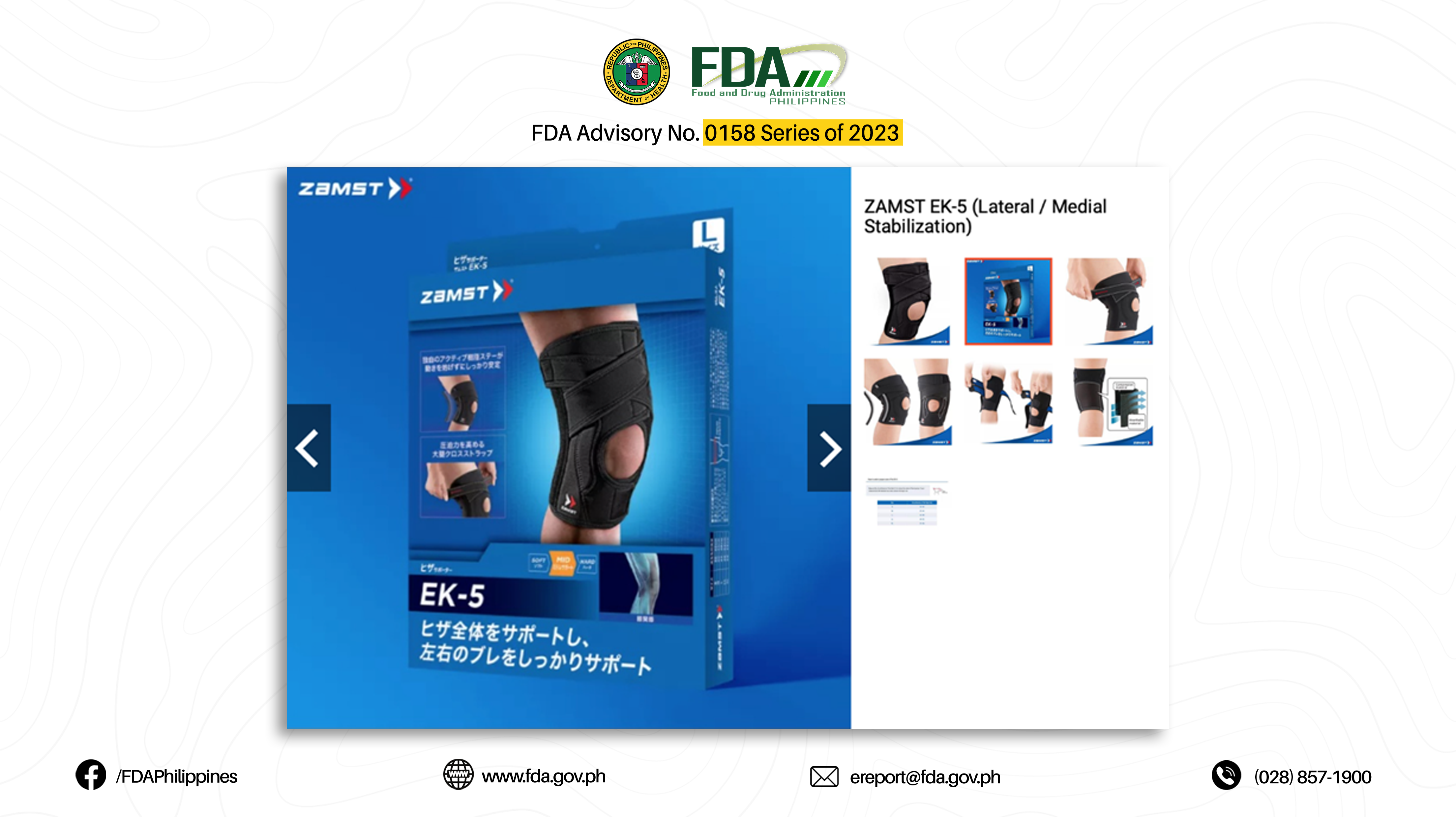
25. “EK-5 (LATERAL / MEDIAL STABILIZATION)”
26. “SHOUDER WRAP (LIGHT COMPRESSION AND PROTECTION)”
27. “BODYMATE CALF (LIGHT COMPRESSION AND PROTECTION)”
28. “CALF AND ANKLE SLEEVES (COMPRESSION AND PROTECTION)”
29. “FILMISTA CALF (NEW GENERATION ULTRATHIN CALF SUPPORT)”
30. “FILMISTA WRIST (NEW GENERATION ULTRATHIN WRIST SUPPORT)”
31. “ELBOW BAND (FOR ELBOW TROUBLE WITH GOLF AND TENNIS)”
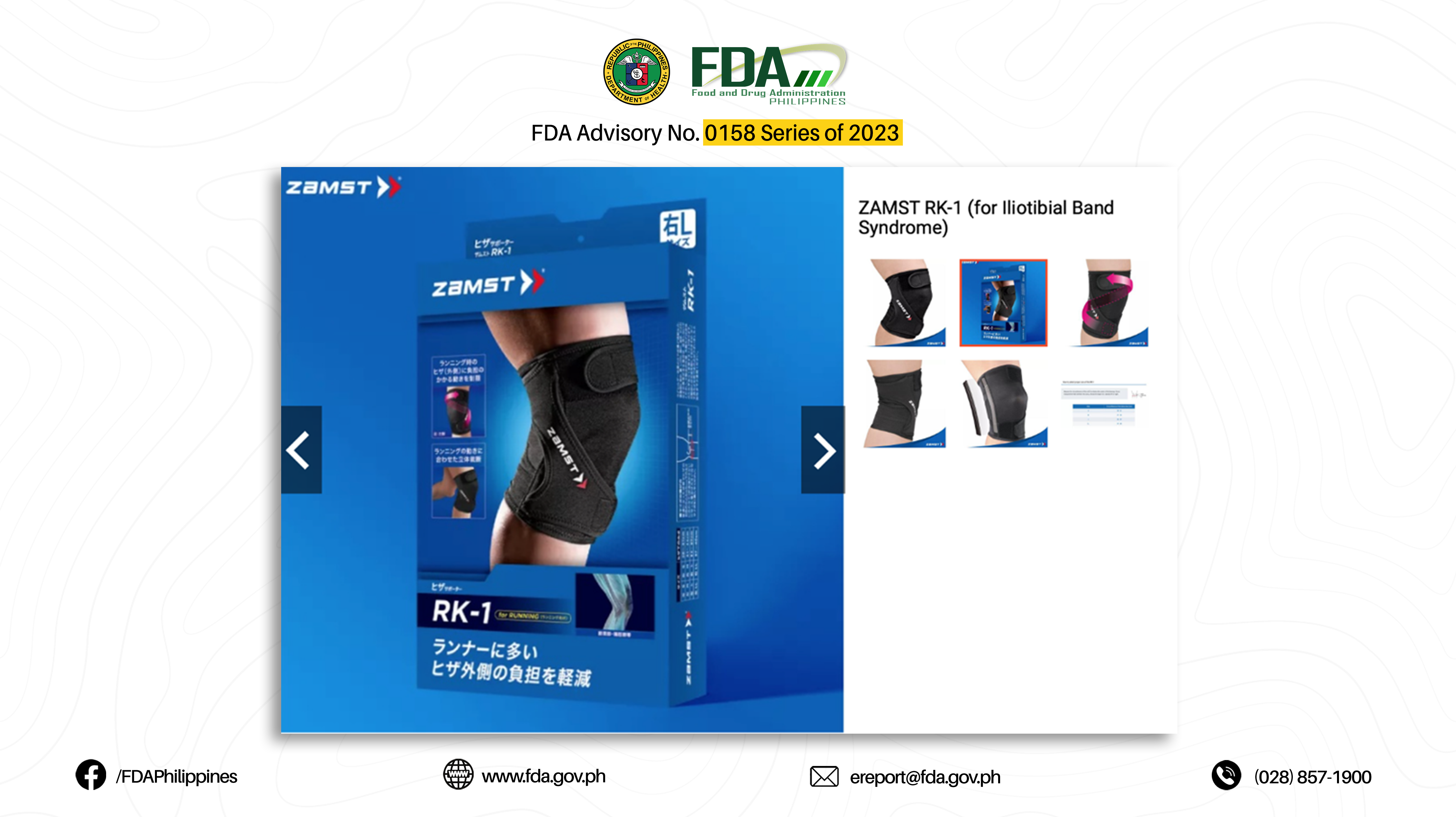
32. “RK-1 (FOR ILIOTIBIAL BAND SYNDROME)”
33. “BODYMATE ANKLE (LIGHT COMPRESSION AND PROTECTION)”
34. “AT-1 (FOR ACHILLES TENDON)”
35. “ZW-7 (STRONG AND FIRM BACK SUPPORT WITH 3D BACK PANEL)”
36. “ELBOW SLEEVE (SLEEVE-STYLE ELBOW SUPPORT)”
37. “FOOTCRAFT CUSHIONED FOR SPORTS (FUNCTIONAL INSOLES)”
38. “ZK-3 (FOR SPRAINS OF THE LCL AND MCL)”
39. “IW-1 SET (ICING SYSTEM WITH 1 ICE BAG)”
40. “CALF SLEEVES (COMPRESSION AND SUPPORT)”
The FDA verified through post-marketing surveillance that the above mentioned medical device products are not notified and no corresponding Product Notification Certificates have been issued. Pursuant to the Republic Act No. 9711, otherwise known as the “Food and Drug Administration Act of 2009”, the manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising or sponsorship of health products without the proper authorization is prohibited.
Since these unnotified medical device products have not gone through evaluation process of the FDA, the agency cannot assure its quality and safety.
All concerned establishments are warned not to distribute, advertise, or sell the said violative medical device products until the Product Notification Certificates are issued, otherwise, regulatory actions and sanctions shall be strictly pursued. Always check if a product has been notified with the FDA before purchasing it by making use of the embedded Search feature of the FDA website accessible at www.fda.gov.ph. You may also look for the FDA Registration number on the product label in the form of CMDN-xxx.
All Law Enforcement Agencies (LEAs) and Local Government Units (LGUs) are requested to ensure that these products are not sold or made available in the market or areas of jurisdiction.
The Bureau of Customs is urged to restrain the entry of these unnotified products.
For more information and inquiries about this advisory, kindly contact the FDA CDRRHR through e-mail at [email protected] indicating on the subject the concerned Advisory, or call (02) 8857-1900 loc. 8301.
To report any sale or distribution of unnotified medical device, contact the online reporting facility eReport through e-mail at [email protected].
Dissemination of this advisory to all concerned is hereby requested.