The Inter-Agency Task Force for the Management of Emerging Infectious Diseases has issued Resolution No. 160 Series of 2022 dated 03 February 2022 wherein it was resolved, among others, that COVID-19 vaccine clinical trial applications shall now be submitted directly to the Food and Drug Administration. Accordingly, Section IV.4.A of FDA Circular No. 2020-029 is hereby amended as follows:
A. COVID-19 Vaccine and Therapeutic Clinical Trial
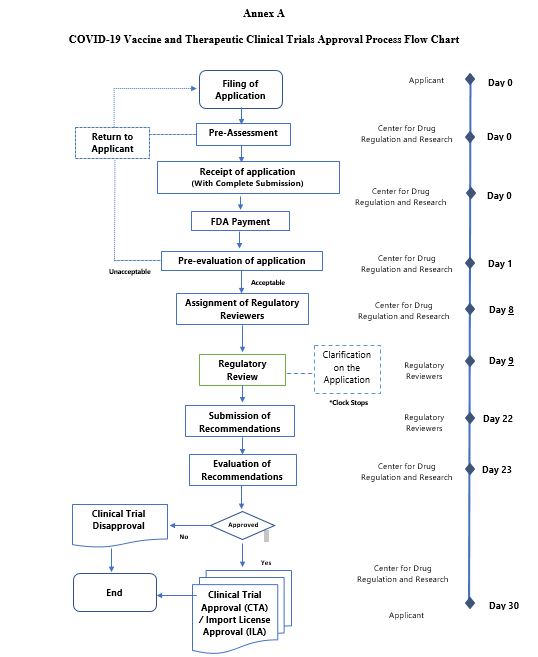
a. A clinical trial for vaccine and therapeutics of COVID-19 shall gain authorization from the FDA for its conduct in the Philippines through the process of approval illustrated in Annex A.
Xxx xxx xxx.
In addition, the Sponsor or CRO shall simultaneously submit an application to their designated Research Ethics Committee (REC) or to the Single Joint Research Ethics Board (SJREB) for multi-site studies with at least one (1) DOH hospital involved. The Sponsor and/or CRO shall follow the existing guidelines of Philippine Health Research Ethics Board (PHREB) and SJREB relative to the conduct of COVID-19 related clinical trials in the Philippines. The decision of the REC/SJREB shall be provided to the FDA.
Section IV.4.B of FDA Circular No. 2020-029 is hereby repealed.
All other provisions of FDA Circular No. 2020-029 not affected are maintained and in effect.
This Circular shall take effect immediately and made coterminous with the duration of the public health emergency due to COVID-19 as declared in Proclamation No. 922 s. 2020, or the state of national calamity as declared in Proclamation No. 1218 s. 2021 further extending the period of the State of Calamity throughout the Philippines until September 12, 2022, unless earlier lifted or extended as circumstances may warrant.