Food and Drug Administration Included in the ASEAN Mutual Recognition Arrangement (MRA)

PRESS RELEASE 27 DECEMBER 2019 ASEAN Member States officially listed the Philippine Food and Drug Administration (FDA) under the ASEAN Mutual Recognition Arrangement (MRA) on Good Manufacturing Practices (GMP) for […]
FDA Advisory No. 2019-534 || PUBLIC HEALTH ADVISORY ON THE ISSUE ON ALLEGED METHANOL POISONING DUE TO CONSUMPTION OF ‘LAMBANOG’

In view of the on-going issue involving mass hospitalization and deaths allegedly due to methanol poisoning after drinking distilled coconut sap, locally known as “lambanog”, the Food and Drug Administration […]
FDA Advisory No. 2019-533-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Pekeng Bersyon ng Cholecalciferol (Fern-D)

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng pekeng bersyon ng Cholecalciferol (Fern-D): Ayon sa pagsusuri ng FDA kasama ang Marketing Authorization Holder […]
FDA Advisory No. 2019-533 || Public Health Warning Against the Purchase and Use of the Counterfeit Version of Cholecalciferol (Fern-D)
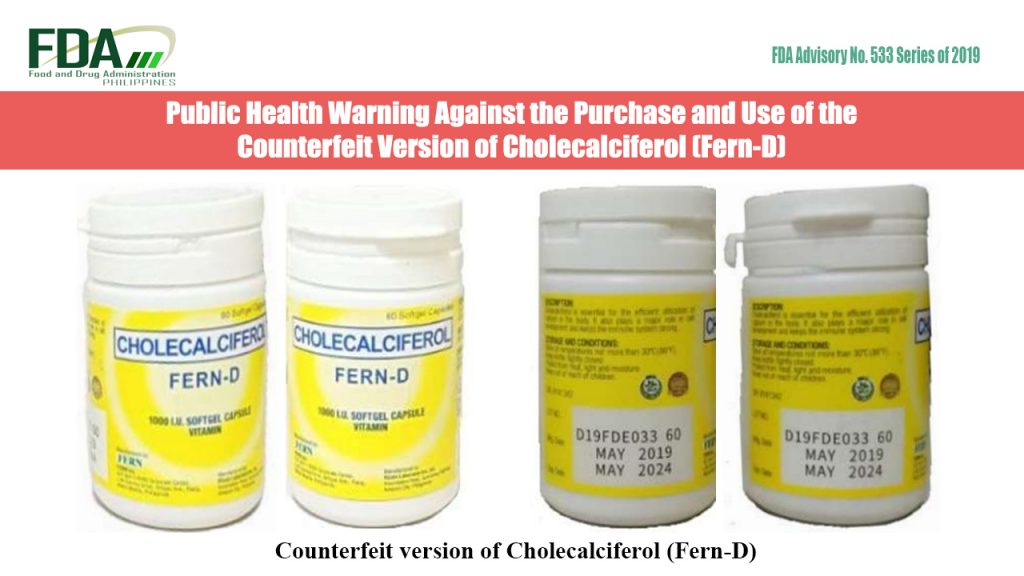
The Food and Drug Administration (FDA) advises the public against the purchase and use of the counterfeit version of Cholecalciferol (Fern-D): The FDA together with the Marketing Authorization Holder (MAH), […]
FDA Advisory No. 2019-528 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device “Flashlight Earpick”

to continue reading, click the attachment below… Attachment:->FDA Advisory No.2019-528
FDA Advisory No. 2019-527 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device “Bio Swiss Cupcake Shaped Bandage”

The Food and Drug Administration (FDA) warns the general public against the purchase and use of the unregistered medical device: “BIO SWISS CUPCAKE SHAPED BANDAGE” The FDA verified through post-marketing […]
FDA Advisory No. 2019-526 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device “Care Touch Oral Medicine Syringe”

The Food and Drug Administration (FDA) warns the general public against the purchase and use of the unregistered medical device: “CARE TOUCH ORAL MEDICINE SYRINGE” The FDA verified through post-marketing […]
FDA Advisory No. 2019-525 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device “Surgitech Clinical Fieber Thermometer MT-101”

The Food and Drug Administration (FDA) warns the general public against the purchase and use of the unregistered medical device: “SURGITECH CLINICAL FIEBER THERMOMETER MT-101” The FDA verified through post-marketing […]
FDA Advisory No. 2019-524 || Public Health Warning Against the Purchase and Use of Unregistered Medical Device “Qinlishu Flex Freely Abacterial Flexible Fabric Bandage”

The Food and Drug Administration (FDA) advises the general public and all healthcare professionals against the purchase and use of the unregistered medical device: “Qinlishu Flex Freely Abacterial Flexible Fabric […]
FDA Advisory No. 2019-523 || Voluntary Recall of Endo GIA Articulationg Reload with Tri-Staple Technology

The Food and Drug Administration (FDA) warns all healthcare professional and the general public on the voluntary recall of Endo GIA™ Articulating Reload with Tri-Staple™ Technology with MDR No. 00768, imported and […]



