
Close

Under Republic Act (RA) 10963, otherwise known as the “Tax Reform for Acceleration and Inclusion or TRAIN Law”, and RA 11534, otherwise known as the “Corporate Recovery and Tax Incentives for Enterprises Act or CREATE Act”, the health products that are exempted from Value-Added Tax (VAT) are medicines indicated for the prevention and management of diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, and kidney diseases, and medicines and medical devices specifically used for the prevention and treatment of COVID-19.
A copy of the list of medicines for diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, and kidney diseases are attached as Annex A. The list of medicines and medical devices specifically used for the prevention and treatment of COVID-19, however, can be accessed at https://doh.gov.ph/VAT-Exempted-Drugs-List.
For convenience, a searchable database is uploaded at https://verification.fda.gov.ph, under the tab “VAT-Exempt Health Products”.
The Department of Health (DOH) and the Food and Drug Administration (FDA) identify which specific medicines are included in the List of VAT-Exempt Health Products, and this List is transmitted to the Bureau of Internal Revenue (BIR).
The FDA is tasked to update and maintain the list of medicines for diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, kidney diseases, and COVID-19 medicines and medical devices. To do this, the FDA regularly updates the list based on the list of registered products with the FDA. Products are included in the respective list based on the indication approved by the FDA.
Following Joint DOF-DOH-BIR-FDA Administrative Order No. 2-2018 dated 21 December 2018 and Joint DOH-DOF-FDA-BIR-BOC Administrative Order No. 2021-0001 dated 23 June 2021, the FDA must provide an updated list thirty (30) days prior the beginning of every quarter. Thus, the FDA endeavors to publish the list within the months of February, May, August, and November of every year.
Requests to add, remove, or correct any entries in the list of medicines for diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, or kidney diseases are submitted through the ‘Enquiry Form for the List of VAT-Exempt Medicines’ at https://bit.ly/vatexemptenquiry. The same form is accessible at the FDA Verification Portal, https://verification.fda.gov.ph. Enquiring entities are requested to complete the following information, in order to appropriately process the query:
a. Generic name
b. Brand name (if applicable)
c. Dosage strength
d. Dosage form and route
e. CPR Number (if available)
f. Indication
g. Type of request (inclusion, delisting, correction)
h. Specific list pertained to in the request (medicines for diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, or kidney diseases)
i. Other details of request (e.g. reason for request, type of correction requested)
Changes to the list are included in the regular quarterly update. Enquiries are consolidated at the end of January, April, July, and October of every year, to allow sufficient time for the publication of the list for the regular quarterly update.
For changes to the list of medicines and medical devices specifically used for the prevention and treatment of COVID-19, enquiring entities are advised to contact the FDA Policy and Planning Service at [email protected].
The list issued by the FDA intends to identify drugs specifically used for the prevention and management of diseases (i.e. diabetes, hypertension, cancer, high cholesterol, mental illnesses, tuberculosis, and kidney diseases) which have been identified under the TRAIN Law and CREATE Act. The fields included in the list have, thus, been so designed to allow the identification of specific drugs which have been registered with the FDA for the purposes of preventing and treating the identified diseases included in the afore-cited laws.
However, it is also acknowledged that drug products containing the same active pharmaceutical ingredients and bearing the same dosage strength and form may remain to be indicated for multiple diseases. In such cases, a distinction can be made when such drugs are used for the management of a disease other than those identified in the aforecited laws, ergo if a product is not specifically prescribed for the treatment and/or prevention of the identified diseases, it should not be construed to be among the medicines which are considered VAT-Exempt pursuant to the TRAIN Law and the CREATE Act.
Please be also informed that the FDA is currently undertaking a review of the drug registry database to address this in the long-term, with the aim of improving the accuracy of reflected products in the published list of VAT-Exempt Products.
No, the fields included in the tabulated list generated by the FDA were so designed in order to identify specific drugs which have been registered with the FDA for the purposes of preventing and treating the identified diseases included in the TRAIN Law and CREATE Act. Thus, if the product bears a dosage form not included in the List, it is NOT considered as the same as the entry.
Further note that active pharmaceutical ingredients of such drugs can be indicated for multiple diseases, and so in recognition of this, the FDA included the dosage forms of the drug products to accurately produce a list which fits the intention of the provisions of the afore-cited laws. However, as noted in FAQ No. 5, there remains to be circumstances which allow for further distinction.
The FDA does not certify if products are considered VAT Exempt. For the purposes of availing VAT Exemption, stakeholders are advised to take reference to the List published by the FDA along with their copy of the Certificate of Product Registration. Should there be any discrepancy, entities are advised to submit their queries following FAQ No. 4.
LTO is an authorization issued by the FDA to an establishment to grant permission to undertake a trade or carry out a business activity, such as manufacturing, importation, exportation, sale, offering for sale, distribution, or transfer of food products.
A sole proprietorship, a partnership, a corporation, an institution, an association, or an organization engaged in any of the following activities are being licensed by FDA:
E-Portal System
The applicant company must request for a user account to FDA Action Center (FDAC) by sending an email to [email protected] with the ff. format:
SUBJECT: Request for eLTO User Account
BODY:
a) Email address (preferably company email address)
b) Name of the Authorized company representative (preferably permanently employed and not merely a consultant)
c) Position in the company
d) Contact Number
E) Company Name
Then, the applicant company must attach a notarized Authorization letter for the eLTO User Account Application (please refer to ANNEX B of FDA Circular no. 2016-004 (Procedure on the Use of the New Application Form for LTO thru the FDA e-Portal)
After issuance of user account and password, the applicant company can access the e-Portal through https://eportal.fda.gov.ph for filing of application
E-Services System
The eServices Portal is accessible through this link http://eservices.fda.gov.ph/. For a step by step procedure, please refer to the Annexes of the FDA Circular 2021-012 .
Creation of account and password is no longer a requirement in the E-services system. Instead, the applicant company will declare its email address then the system will automatically send notifications to the applicant company’s email address.
Reminder: the declared email address is unalterable. The applicant company shall make sure that the email address is within the scope and access of the Authorized Person/s, Qualified Personnel and/or owner of the establishment
For system concerns, please send an email to [email protected] for assistance.
In the ePortal system, the case application should be under the “PARTICIPATED” folder of the applicant company. If the case application is still in the “DRAFT” folder, the applicant company should go over the case application and click “CONTINUE” to assign the task to payment.
In the eServices system, the Application Summary shall be automatically sent to the applicant’s registered e-mail address to indicate the successful submission of the application.
In the ePortal System, the applicant company should download the Order of payment and pay the prescribed fees indicated in the Order of payment.
In the eServices System, the application will be pre-assessed with regards to completeness and correctness of the application vis-à-vis uploaded requirements. Applications with incomplete documentary requirements and/or inconsistent data entries with the submitted documentary requirements based on existing FDA rules and regulations shall not be accepted and the application will not proceed to the next step of the process. The applicant company will be notified on the reason/s of disapproval via declared email. If the application passed the pre-assessment step, the applicant company shall receive the Order of Payment with Reference Number through declared e-mail indicating the fees to be paid.
The result of the application shall be sent to the applicant’s registered e-mail.
For eServices System, the eLTO will be printed by the applicant company on a standard A4 size (21 cm x 29.7 cm) paper, on full-colored page and in portrait orientation. The printed eLTO shall be positioned on the most conspicuous place within the business establishments. A QR Code verifier shall be included in the LTO as means of confirmation of the legitimacy of the document.
The grounds for disapproval of LTO application may be any of the following, as stated in AO No. 2020-0017 and AO No. 2014-029;
The disapproval of an application is without prejudice to re-application. However, disapproval shall mean outright forfeiture of payment.
No. Variation and renewal applications must be applied separately. If a Food Product Establishment is due for renewal but is expected to apply for changes in information that need to be reflected in the system or registry, then a renewal application must first be submitted. . In addition, the applicant company cannot apply for a renewal of application if not within ninety (90) days before the expiration date of the LTO.
The following documents based on AO 2020-017 shall be uploaded using the E-Portal. Scanned copy should be 100-150 dpi (dots per inch), maximum of 2MB:
Initial LTO Requirements:
Any one of the following shall be submitted as proof of business name registration (in pdf):
Site Master File (SMF), which shall be required for applicants applying for LTO as manufacturers of large and medium food manufacturers (CFRR)
Additional Requirements, as applicable based on FDA Circular 2021-012 (Food Traders/Distributors):
LTO Renewal Requirements:
Note: Application for renewal shall be done within three (3) months prior to the validity date of the LTO. Applications filed after the validity date of the LTO shall be subject to surcharge as prescribed in RA 9711 and its IRR.
Requirements for Specific Variation in the LTO
Major Variation for Local Manufacturers
| Type of Variation | Requirement |
| Transfer of Location of Manufacturing Plant
- Physical transfer of the establishment (and may entail changes in the previously approved address) |
a. Business permit reflecting the new address
b. Updated Site Master File to be presented upon inspection |
| Expansion of Manufacturer and/or Additional Product Line; or Change of Manufacturing Activity
- Expansion shall refer to expansion made which is adjacent to the existing location of the establishment - Additional product line refers to additional type or class of products produced within the same manufacturing site - Change in manufacturing activity shall refer to an additional activity that a manufacturer engage in (e.g. LTO as Manufacturer with additional activity as Repacker) |
Updated Site Master File to be presented upon inspection |
Minor Variation
|
A Qualified Person refers to an organic or full-time employee of the establishment who possess technical competence related to the establishment’s activities and health products by virtue of his profession, training or experience. A qualified person has the responsibility to comply with the technical requirements of the FDA or discuss or clarify matters with the FDA when submitting technical requirements or engage the FDA officials when conducting inspection or post-market surveillance activities. The qualified person may also be the duly Authorized Person of the establishment.
| Qualification | Training Requirement |
| Company Regulatory Officer (Authorized Person) and Food Safety Compliance Officer who is preferably a graduate of food-related courses including but not limited to food technology, food and nutrition, chemistry, microbiology, chemical/sanitary engineering, veterinary medicine, fisheries, agriculture (RA 10611) | Certificate of Attendance on seminar on food safety, GMP or HACCP given by the academe, WHO, FAO, NGOs, cooperative, food industry organizations, professional organizations, or the FDA Academy |
Initial LTO has a validity of 2 years, while renewal is valid for 5 years.
For bottled water, renewal is valid for 3 years
| Classification |
Initial (2 years validity) |
Renewal
(5 years validity) |
|
| 1. Food Distributors
(Importer, Exporter, Wholesaler) |
8,080.00 |
20,200.00 |
|
| 2. Food Manufacturer/Trader | |||
| 2.1 250K and below |
1,010.00 |
2,525.00 |
|
|
2.2 Over 250K but below 500k |
1,515.00 |
3,788.00 |
|
| 2.3 500K but below 1M |
2,020.00 |
5,050.00 |
|
| 2.4 Over 1M but below 5M |
4,040.00 |
10,100.00 |
|
|
2.5 5M but below 10M |
6,060.00 |
15,150.00 |
|
| 2.6 10M but below 20M |
10,100.00 |
25,250.00 |
|
| 2.7 20M but below 50M |
20,200.00 |
50,500.00 |
|
| 2.8 50M and above |
30,300.00 |
75,750.00 |
|
| 3. Bottled Water Manufacturer/ Trader/ Distributor |
3,030.00 |
5,050.00 (3 years validity) |
|
| 4. Iodized Salt Manufacturer/Trader | |||
| 2.1 Large and Medium |
2020.00 |
5,050 |
|
| 2.2 Small |
1010.00 |
2,525 |
|
| 2.3 Subsistence |
410.00 |
1,010 |
|
|
5. Iodized Salt Distributor |
2,020 |
5,050 |
|
Note: Surcharges/penalties are imposed for late renewal as per FDA Circular 2011-004.
Based on FDA Advisory 2021-0246, please refer to the below payment options:
|
Payment Option |
Credit |
|
via Landbank ATM Card |
Real-time Credit* |
|
Other Banks via Bancnet |
Next banking Day* |
| Cash Payments via 7/11 and Bayad Center |
Next Banking Day* |
| *for transactions made during holidays and weekends, payment will be credited on the next banking days | |
Further, FDA is now included in the Landbank Link.BizPortal as biller:
https://www.landbank.com/e-banking/other-e-banking-services/linkbizportal
Applicant companys are also hereby advised that payments through FUND TRANSFER via account number 0392-1030-58 will NO LONGER be accommodated effective immediately since Landbank Link.BizPortal is now available.
The LTO shall be renewed within 90 days before its expiration as per Administrative Order 2020-017
An application for renewal of an LTO received after its date of expiration shall be subject to a surcharge or penalty equivalent to twice the renewal licensing fee and an additional 10% per month or a fraction thereof of continuing non-submission of such application up to a maximum of 120 days.
An application for renewal of license filed after 120 days shall be considered expired and the application shall be subject to a fee equivalent to the total surcharge or penalty plus the initial filing fee and the application shall undergo the initial filing.
Kindly refer to the revised Citizen’s Charter for the prescribed timeline per type of LTO application:
Please send an official letter to [email protected] for cancellation of LTO and/or closure of establishment.
Please send an email to [email protected] and/or [email protected] for payment concerns i.e posting of payment, refund/transfer of payment, etc.
Please send an email to [email protected] and/or [email protected] for technical inquiries
All Food Manufacturers (Importer of raw material for own use/ Exporters).
Food Establishments may apply for issuance of GMP Certificate after at least one (1) year of operation with a valid LTO
Based on FDA Circular 2020-026, the FDA Action Center (FDAC) receives the letter request for issuance of GMP/HACCP Certificate via fdac.letters@fda,gov.ph. The FDAC personnel will respond to the email and provide to the applicant company the designated document tracking number of the application.
| Requirements | Where to Secure |
| a) Inspection report with certificate of Compliance/ Recommendation Letter from RFO | FDA Regional Field Office |
| b) Valid LTO | Center for Food Regulation and Research |
| c) Proof of payment | FDA Cashier/ Other FDA Authorized Payment Portals/ Banks |
| Authorization | Fees |
| a) cGMP | P500.00 + LRF per year |
| b) HACCP | P1,000.00 per product + LRF per year |
Unless the LTO is revoked, validity of GMP Certificate shall be coterminous with the LTO validity, whereas the HACCP Certificate shall only be valid for one (1) year.
All Food Manufacturers of Fortified Products.
Based on FDA Circular 2020-026, the FDA Action Center (FDAC) receives the letter request for issuance of GMP/HACCP Certificate via [email protected]. The FDAC personnel will respond to the email and provide to the applicant company the designated document tracking number of the application.
Basic Requirements based on RA 8976 (Food Fortification Law of 2000), RA 8172 (ASIN Law), the Sangkap Pinoy Seal Program Manual of Operations (December 2000) and Administrative Order No. 82 s. 2003 (Guidelines on the Granting of Diamond Sangkap Pinoy Seal to Manufacturers of Fortified Products):
| Requirements | Where to Secure |
| a) Duly accomplished application forms | FDA Website |
| b) Valid LTO | Center for Food Regulation and Research |
| c) Results of product analysis for vitamin A, Iron and Iodine from an FDA recognized laboratory. | Laboratory analysis issued/conducted by FDA-Recognized laboratories. |
| d) Sample label with Sangkap Pinoy Seal/ Diamond Sangkap Pinoy Seal | Applicant Company/ Manufacturer/ Source/ Supplier |
| e) Proof of payment | FDA Cashier/ Other FDA Authorized Payment Portals/ Banks |
| f) Inspection report with Certificate of Compliance | FDA Regional Field Office |
| Authorization | Fees |
| a) Use of the seal (Regular Seal) | P8,000.00 non-refundable fee + 1% LRF |
| b) Processing fee for every application (Regular Seal or Diamond Seal) | P500.00 + 1% LRF |
Unless the LTO is revoked, validity of Diamond Sangkap Pinoy Seal/ Sangkap Pinoy Seal Certificate shall be valid for an indefinite period.
For pre-assessment, no specific timeline is committed in the Citizen's Charter. However, our initial target is to process applications for pre-assessment within 7 WORKING DAYS depending on the bulk of applications received per week.
New case should be created selecting the previous application's type (eg. Initial, Renewal, Reapplication or Amendment) instead of reapplying the denied pre-assessment.
Denied Initial application on E-portal V2 should be applied on the same case number instead creating a new case unless declared on the issued Letter of Denial.
No. Any change from the initially approved application, except for changes equivalent to initial application, shall be filed as amendment (e.g. compliance to CPR remarks, and other post-approval changes as per FDA Circular No. 2020-033) prior to filing of renewal application.
No. Renewal should be applied first before applying for an amendment. Amendment should be filed on the same case number used in the renewal application.
A system-generated email notification of complete requirements will be sent to the registered E-mail. On the other hand, you may also download the result of pre-assessment under the Generated Documents tab in the case number. Right click on the case number. Select "Summary" and look for the Generated Documents tab. Once the document appears, tick the file then click download.
All processed food and food products including food additives, food supplements and bottled water manufactured and/or distributed (i.e. imported, exported and/or wholesale for local distribution), for trade and/or repacked are required to secure a Certificate of Product Registration before these are sold, offered for sale or use, distributed or supplied, among other marketing and promotional activities as per R.A. 9711 and A.O. 2014-0029.
Food establishments with License to Operate as Manufacturer and Trader who directly import and use raw materials, ingredients and food additives for their own use or for further processing to manufacture a processed food product, need not secure a CPR for the raw materials, ingredients and food additives. However, the sources of these raw materials should be notified to FDA to be reflected in the Licensing Database of Center for Food Regulation and Research.
You may refer to FDA Circular No. 2020-033 “Procedure for the Use of the Modified Electronic Registration System for Raw Materials and Prepackaged Processed Food Products Repealing FDA Circular NO. 2016-014 “Procedure for the Use of Electronic (E-Registration) System for Pre-Packaged Processed Food Products” to include Guidelines for Pre-assessment and Reiteration of Pre-Assessment Procedures in Applying for Certificate of Product Registration for Food prior to filing for registration. For further guidance on registration requirements and food regulations, please refer to Administrative Order No. 2014-0029 (Rules and Regulations on the Licensing of Food Establishments and Registration of Processed Food, and Other Food Products, for Other Purposes). You need to fill up Initial Application Form and upload a scanned copy of the following documents in the system during application:
As applicable, documents to substantiate claims, such as technical, nutritional or health studies or reports, market-research studies, Certificate of Analysis, quantitative analysis and computations, scientific report or studies published in peer-reviewed scientific journals, certificates or certification to support use of logo/seal on Sangkap Pinoy, Halal, Organic, or Kosher food and in compliance with current labelling regulations.
All applications for food product registration shall be lodged in the Modified E-portal version 2 (https://eportal.fda.gov.ph) and shall undergo pre-assessment (FDA Circular 2020-033-A).
All food establishments applying for product registration should have a valid License to Operate issued by FDA. For locally produced products, the product being applied should be included in the LTO of the manufacturer.
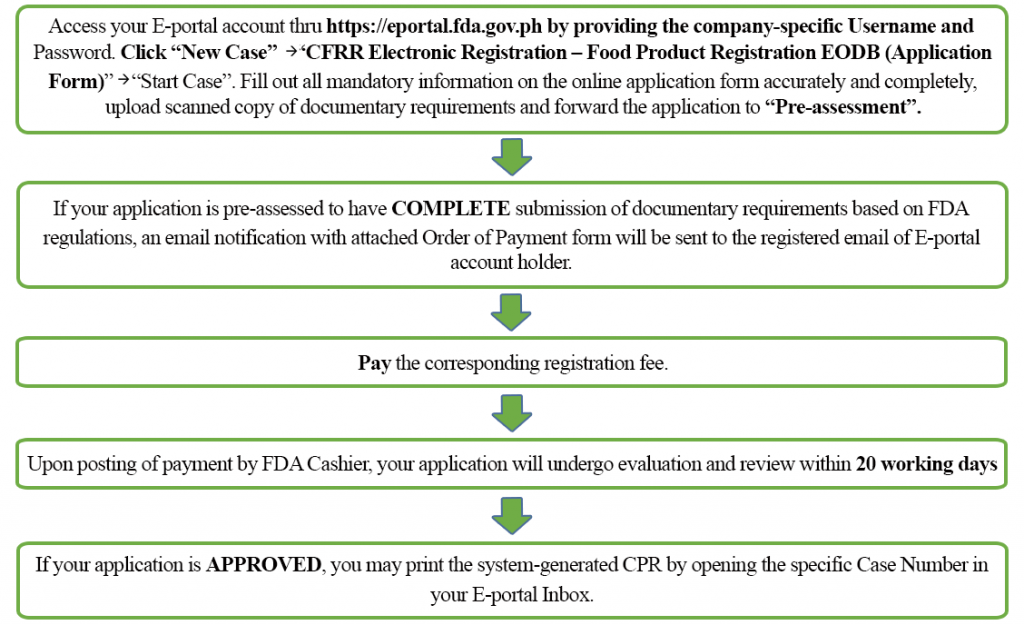
However, if your application for pre-assessment is found to have INCOMPLETE submission of documentary requirements based on FDA regulations, an email notification with attached Results of Pre-assessment will be sent to the registered email of E-portal account holder. The Case Number will close upon assessment of initial, amendment, renewal or reapplication data capture applications having incomplete submission of documentary requirements. You will then need to upload again ALL previous and current documents and file a new application using a new case number. You may refer to FDA Circular No. 2020-033-A for the detailed guidelines on pre-assessment procedures in applying for CPR for food in line with FDA Circular No. 2020-033.
If your successful pre-assessed application is DENIED, an email notification with attached Letter of Denial will be sent to the registered email of E-portal account holder. You may also download the LOD by opening the specific case number in your E-portal Inbox. File reapplication on the same case number, upload scanned copy of documents in compliance to the mentioned deficiencies and forward it to “Pre-assessment”. If your reapplication is pre-assessed to have COMPLETE submission of documentary requirements based on FDA regulations, an email notification with attached Order of Payment form will be sent to the registered email of E-portal account holder. Pay the corresponding reapplication fee. Otherwise, you will receive an email notification with attached Results of Pre-assessment. Re-file the reapplication on the same case number, upload scanned copy of documents in compliance to the mentioned deficiencies and re-forward it to “Pre-assessment”.
You may also refer to the video on Complete Guide on How to Apply for Food Certificate of Product Registration Using E-portal Version 2
Fees and charges will be computed by the system based on the number of years (2, 3, 4 or 5 years validity) applied for and type of product. You may refer to Administrative Order No. 50-2001 for the schedule of fees. Payment may be made through the FDA Cashier by presenting your printed Order of Payment form. Payments through Bancnet Online and LandbankOncoll are also accepted.
Only Initial applications for Food Supplement are required to submit a representative sample in commercial presentation consistent with the E-Registration application. It should be properly labelled with the respective case number, packaged accordingly to protect the contents and submitted to the Food and Drug Administration Main Office Building within ten (10) days upon payment of assessed fee through either of the following means:
-TO: FOOD AND DRUG ADMINISTRATION – Civic Drive, Filinvest City, Alabang, Muntinlupa City 1781
-FROM: Company’s complete name and address
-SUBJECT: Food Product E-Registration Application (Case No.)
-Brand Name
-Product Name
-Email address
A previously registered product initially for local distribution shall be allowed to be exported using the same CPR as long as the following conditions are met, and labelling and standards of the importing country are likewise met:
The same brand name
The same product name/variant
The same product formulation/ ingredients in the same order of proportion
Amendment application of Packaging Design for export product shall be made and labels in the language of the importing country shall likewise be submitted.
Applications filed through the E-Registration system are processed within 20 working days. You may track the applications through the Process Map function of the system in the electronic portal.
Yes. Each importer should secure his/her own CPR for the products that he/she will import. The name and address of the importer is reflected on the CPR which is needed during importation and release of goods from the Bureau of Customs. The CPR also authorizes the importer/distributor/wholesaler to distribute the products locally.
The CFRR E-Registration User Account and Password is company-specific. An authorized representative handling multiple companies must secure a separate user account and password for each respective company. You must secure a notarized authorization letter from the company (with a valid License to Operate Number) being represented or the company account holder. Send the request for a User Account to [email protected] following the format specified below with the scanned notarized authorization letter:
SUBJECT: CFRR:E-Registration
BODY: Email Address:
Last Name:
First Name:
Middle Name:
Company Name:
The issued CFRR E-Registration User Account will be sent to the e-mail address you provided in the request. Be sure to check the Spam folder of your e-mail if you have not received a reply within 3 working days.
You may also use your E-LTO User Account and Password for E-Registration by having it validated through a request sent via email to [email protected] following this format:
SUBJECT: CFRR:E-Registration (Re-validate)
BODY: Email Address:
Last Name:
First Name:
Middle Name:
Company Name:
User Account:
Password:
The User Account and Password validity is the same with the issued License to Operate (LTO). You will need to renew these by sending a request via email to [email protected] following this format:
SUBJECT: CFRR: E-Registration (Renewal of User Account & Password)
BODY: Email Address:
Last Name:
First Name:
Middle Name:
Company Name:
User Account:
Password:
You may refer to FDA Circular No. 2020-033 for other details and format of documents required in securing a User Account and Password for E-Registration.
Yes. This procedure is for Data Capture only wherein it is intended for applications with existing CPR and denied applications in the old (version 1) of E-Registration. Provide the Food Registration Number and Old Case Number under General Information for Amendment and Renewal. In reapplication, declare the old application number only under General information step.
Applications under Notification of Amendment have no changes in CPR information (except CPR remarks, if applicable) but will still automatically generate CPR under Generated Documents Tab. Once the case number is already in your inbox account, you can click the case number and view the Amendment Summary Table which reflects the result of your application. If the application is disapproved, you can see the remarks or reason for its disapproval on the right side of the table.
There is a new Food Categorization in the system under High Risk HRK2: Herbs and botanicals and/or Products with other nutritional substances and/or combination as Conventional Food Product. This new categorization is not yet specified in the Annex A of FDA Circular No.2016-014 and Administrative Order No.2014-0029 Annex A.
Same as the requirements for conventional food products as stipulated in the Administrative Order 2014-0029 with the addition of stability study, Certificate of Analysis (COA) of the finished product and as applicable, safety data (e.g. LD-50 Toxicity Test). For food supplements containing herbs which are not listed in any official pharmacopeia and substances with no established safe levels.
Vitamins and Minerals levels are computed by % RENI, as per Office Order 22 s.1991.To be classified as food supplement, the maximum limit is 150% RENI for Water Soluble Vitamins and 105% RENI for Fat Soluble Vitamins. Herbals and Botanicals that cannot be computed since it has no % RENI requires a justification for the safety and test for toxicity study (LD50 Toxicity Test) and/or inclusion in the GRAS list or Official Pharmacopeia Listing.
Either Dietary Supplement or Food Supplement is acceptable to use as per the definition of Dietary/Food Supplement on the RA 9711. Herbal Dietary Supplement maybe allowed for food supplement containing multiple herbs but not “Herbal Supplement”.
The Stability Data of the shelf life study should include: conclusion parameters used and methodology declaring the Product name, Batch number, Production date, dates of analysis, Tabulated data & results in terms of physical and chemical and Name and signature of the QA Analyst and QA Manager. We accept results for actual and accelerated shelf life study. If the shelf life study is on-going, results for at least 6 months is allowed.
No, but the levels of Vitamins & Minerals should conform with Office Order 22 s.1991 which sets the limit for vitamins and minerals to be classified as Food Supplement.
If a product is for institutional use where the “institution” is the sister company which has a separate licensed entity, the product should have a separate CPR.
If not company owned, either the manufacturer or distributor should register the product.
If the raw material is a specific additive, you may refer to FAO/WHO JECFA for specification on the limits for heavy metals. You may also refer to Food Chemical Codex for specifications of raw materials on limits for heavy metals.
In the absence of national standards, FDA Philippines only uses applicable Codex Alimentarius standards. As per Republic Act 10611 otherwise known as the Food Safety Act of 2013, Codex standards shall be adopted except when these are in conflict with what is necessary to protect consumers and scientific justification exists for action taken.
Open the case, go to information, and click process map.
Sending additional files to [email protected] is not acceptable. The pre-assessor will assess the application based on the completeness to the requirements specified on FDA Circular No. 2020-033, FDA Circular No. 2020-033-A and Administrative Order No. 2014-0029. The result of pre-assessment will be automatically sent to the registered email of the account holder. Make sure that all attachments are complete before finishing the case/application. The evaluator will assess your application based on the uploaded requirements in compliance with FDA Circular No. 2020-033 and AO 2014-0029. Any additional documents that are submitted after filing of applications shall not be considered.
You may call us at 857-1900 loc. 8105 or 8115, or send an email to [email protected].
Yes, a clear, readable, complete artwork or proposed label may be submitted when you apply for product registration.
No, labels of products exclusive for export market only shall comply with the existing labeling regulations of the importing country or country of destination of the products.
Labels of products for local distribution should conform to the local labeling regulations as stipulated in Administrative Order 2014-0030, while the labels for export market should follow the existing labeling regulations of the importing country. A separate label for export market must be printed in order to comply with the labeling regulations of the country of destination of your products.
No, labels of imported products shall declare the corresponding English translation of mandatory label information. A provisionary sticker can be used for a maximum of six months only. Labels of products in the market should be compliant with the labeling regulations.
One label/artwork showing the principal display and side panels for each of the different packaging sizes of a product should be submitted. Pictures of the product in commercial presentation, from at least 2 different perspectives must also be submitted.
You need to file for an amendment application of the CPR (Change in/additional label design) in the e-portal. You need to upload a letter of intent stating the changes made on the label and the revised label. An amendment fee of PhP 210.00 must be paid.
No, you cannot use the brand name of products previously registered with FDA under a different company unless you have authorization from the brand name owner.
No, the label of your product must declare expiry/expiration date or use by date or consume before date in day/month/year format with the name of the month spelled out, as stipulated in Administrative Order 2014-0030.
If the product has multiple SKUs with different designs but with the same formulation, manufacturer, brand name and product name including description, you only need to upload all labels of different packaging sizes on your application.
Coloring substances shall be declared by their common name or as “Food Color(s)” or “Color(s)” for those that are derived from or identical with substances derived from plant materials, and as “Artificial Color(s)” for coal-tar dyes or other synthetic chemical compounds.
Reference: AO 2014-0030
YES. Stickering is allowed, provided that the sticker is durable and not easily removed. The label information should be printed on, or on a remedial sticker on the label itself and it is only allowed for six months from the date of approval.
Recommended usage is subject for approval during evaluation based on the safety of ingredient/ combination of ingredients in the Herb/Botanical Food Supplement Product.
Yes. However, the importing company must have a valid License to Operate as Food Importer, and supporting documents must be submitted during application as proof that the product is exclusively imported or manufactured for that particular Importer.
Yes. All labels must carry a corresponding English translation of the mandatory labeling information written in foreign language, as stipulated in Administrative Order 2014-0030.
YES. You only need to ensure the durability of the sticker material.
Yes. All prepackaged, processed food products must follow the labeling regulations.
This is the current RENI adopted by the FDA as per Bureau Circular No. 16 s. 2005
Yes. However, the corresponding RENI percentage is not mandatory for imported products but all 9 nutrients must be reflected even if it has no value but need to include “0” or “-“.
The requesting party needs to submit the hard copy of the following documents to FDAC:
The requesting party should submit the hard copy of the following documents to FDAC:
MARIA SOLEDAD Q. ANTONIO, MD, PhD, MPH, DPAFP, CESE
Director IV
Bureau of International Health Cooperation
After the clearance is issued to the requesting party, they should contact the FDA-Regional Field Office at [email protected] for the schedule of inspection. The shipment will not be released without inspection from FDA-RFO.
Reference: DOH Administrative Order 2020-0001
The FDA-Regional Field Office handles this type of request.
The limit is as follows:
Reference: BOC and DOH-FDA Joint Circular No. 1 dated 22 June 2015
No, you just have to notify the Center for Food Regulation and Research (CFRR) on the product’s change in alcohol content and/or vintage.
The following documents should be submitted:
An FDA-CFRR issued letter will be presented to BOC together with the valid CPR to facilitate the release of wines. Reference: FDA Circular 2014-022
This type of clearance is processed by the FDA Central Laboratory and signed by CSL-Director. You may email [email protected] for this type of requests.
As per FDA Memorandum 2020-023, issuance of permit to carry/mail personal items is under the purview of FDA Action Center (FDAC). Submit this type of request to [email protected].
You need to submit complete requirements as follows:
The requesting party should have a valid License to Operate and Certificate of Product Registration to be able to join the exhibit abroad and Payment of Php510.
All promotional campaigns/announcements for consumer products, services, credit facilities which include sponsorships of games shows and similar activities.
All processed food and food products (complete list is in AO 2014-0029) including:
FOOD Category (Imported & Locally Manufactured)
* To be referred to I.A.C–DOH
>>Marketing and practices covered by the Milk Code shall be forwarded to IAC Secretariat to be deliberated by the IAC on EO 51.
However, only food products duly registered with FDA are allowed to conduct sales promotional activities.
Private entities in joint project/s with any government agency under the preceding paragraph
Social, civic, political, religious, educational, professional and other similar organizations) which extend promotional activity among their members. Provided that the promotional activity is not considered sales promotion campaign as defined under these Rules
Reference: DAO No. 2, Series of 1993, as amended by DAO No. 10-02, Series of 2010
Client must email [email protected]. The subject of the email shall follow the following format:
CFRR SALES PROMO PERMIT APPLICATION [Application Type], [Company Name]:
E.g. CFRR SALES PROMO PERMIT APPLICATION INITIAL APPLICATION, ABC COMPANY
For Initial Application
Integrated Application Form
Accomplished Information Sheet and Mechanics of the Promotion
Copy of valid CPR
Advertising/ Collateral Materials to be used in the Promotion, if any
Proof of Payment
*Proof of Schedule from FDAC must also be presented
For Amendment
Integrated Application Form
Letter of Intent stating the desired changes
Copy of approved permit
Additional Advertising/ Collateral Materials to be used in the Promotion, if any
Proof of Payment
Link: List of Acceptable and Not Acceptable requirements for Initial and Amendment Application
Submit requirements to [email protected]. The application will undergo pre-assessment based on the completeness and correctness of the requirements. Proof of Schedule from FDAC must also be submitted. Otherwise, the submitted application will not be pre-assessed.
If the requirements are complete and compliant, an email will be sent to the company with instruction to proceed with the payment.
If the application is incomplete, the applicant will be advised to secure a new appointment schedule to submit the application for pre-assessment. The client must request for a new DTN and schedule of appointment.
*A pre-assessment form will be attached on the email.
Submit the requirements, proof of payment and CFRR pre-assessment form to [email protected] through file-sharing platforms. Please wait for the posting and verification of your payment before you submit the documents.
The fee depends on the coverage of the promotional activity and amount of prizes to be won (per DTI-DOH Joint AO No. 1 s. 2000)
| Coverage | Fee |
| NCR only or in several regions in NCR and Nationwide | Php 1, 010 |
| More than one (1) region but excluding NCR | Php 760 |
| Several provinces/ cities/ municipalities within a single region | Php 560 |
| Single province/ city/ municipality | Php 260 |
| Amount of Prizes | Fee |
| 150,000.00 & below - 300,000.00 | Php 1,010.00 |
| 300,001.00 - 500,000.00 | Php 2,020.00 |
| 500,001.00 - 1,000,000.00 | Php 3,030.00 |
| Above 1, 000,000.00 | Php 5,050.00 |
For amendment including extension, the fee is Php 310.00, but depends if the amount of prizes will be increased.
| Products Involved | Concerned Center |
| Drugs, Food, Cosmetics, Device, HHS (or Drug with any categories) | Center for Drug Regulation and Research (CDRR) |
| Food, Cosmetics, Device, HHS (or Device with any product categories excluding Drug) | Center for Device Regulation, Radiation Health and Research (CDRRHR) |
| Food, Cosmetics, HHS | Center for Food Regulation and Research (CFRR) |
| Cosmetics and HHS | Center for Cosmetic Regulation and Research |
Processing period is seven (7) working days upon receipt of Order of Payment, CFRR pre-assessment form and complete and correct requirements from FDAC.
At least thirty (30) days before the actual commencement of the sales promotion (per Article 116 of R.A. 7394).
The sales promotion campaign shall have a duration of not more than a year, extendible to a maximum of six (6) months upon approval by the department. Price reduction should not exceed three (3) months and Closing out sales should not be more than six (6) months (per Article 116 of R.A. 7394).
As per Book II, Article V of IRR of RA 9711
No. Where such qualification is made in a sales promotion campaign with a period, the sponsor shall be liable therefore throughout the duration of the promotion, whether or not the supply of the products under promotion has been depleted (per Article 116 of R.A. 7394).
Any price reduction promotion on any consumer product or service shall not exceed three (3) months. However, in case of closing – out sales, the period shall be six (6) months (per Article 116 of R.A. 7394).
The status of your application may be checked through the DOCTRACK STATUS function of the FDA website. You may also email [email protected] or [email protected] for follow-ups.
No. Only products with valid CPR may be applied for sales promotion (per RA 9711 Article V Section 2). The company may apply for promotions once the renewed CPR has been released.
Seven (7) working days before the actual activity (per Rules & Regulations Implementing RA 7394).
Email [email protected] and attach letter request and approved sales promo permit.
|
INITIAL APPLICATION |
|||
| REQUIREMENTS |
ACCEPTABLE |
NOT ACCEPTABLE | BASIS |
| 1.Integrated Application Form | -Check the consistency of applicant’s name, establishment’s name and address indicated in the Integrated Application Form
-Check the applicant’s signature |
-Non-submission
-Submitted integrated application form contains incomplete information |
FDA Circular No. 2014-003 |
| 2. Information Sheet and Mechanics of Sales Promotion
|
-It contains 3 sheets and all must be properly filled out
-Promo title -Duration -Coverage -Promo Mechanics
|
-Non-submission
-Submitted information sheet and mechanics of sales promotion contains incomplete information -Applied promo duration has already started or lapsed -With Drug and/or Device product -Duration is beyond 3 months for discount scheme and 6 months for closing out sales -No coverage included -With products covered by the Milk Code or EO 51
|
Republic Act 7394
Executive Order No. 51 or the Milk Code FDA Memorandum Circular No. 2013-028 DTI-DOH Joint Administrative Order No. 01 series of 2000 Codex Stan 180-1991
|
| 3. Duly and validly registered participating products | -Valid Certificate of Product Registration for Food products
-Valid Cosmetic Notification for Cosmetic products*
*In case there are applications with combined food and cosmetic products |
-Expired Certificate of Product Registration for Food products
-Expired Cosmetic Notification for Cosmetic products*
(products undergoing renewal not allowed) |
Republic Act 7394
|
| 4. Promotional Materials, if any
|
-Clear and readable promotional materials
-The line “DOH-FDA-CFRR-Permit No. xxxx s. xxxx must be indicated |
-Not readable promotional materials
- The line “DOH-FDA-CFRR-Permit No. xxxx s. xxxx is not indicated. -With obvious health and nutritional claims and therapeutic claims and/or claims that are not included in the approved label. e.g. Can cure diabetes Can lower blood cholesterol Superior amount of Vitamin C -With sexy tones, skin exposure, potentially controversial/sensitive execution -Use of the FDA Logo and Name - Non-inclusion of the Filipino Standard Message in prescribed format: “Mahalagang Paalala: Ang (name of product) ay hindi gamot at hindi dapat gamitin panggamot sa anumang uri ng sakit” for sales promotion application with Food Supplements as participating products |
Republic Act 7394
Bureau Circular No. 2007-002 FDA Memorandum Circular No. 2013-030 Administrative Order 2010-0008 |
| 5. Proof of Payment of Fees | -Payment details must be posted and verified by the FDA Cashier in the IFS-Document Tracking System | -Non-submission
- Payment details are not posted and verified by the FDA Cashier |
|
| AMENDMENT APPLICATION | |||
| REQUIREMENTS | FOR APPROVAL | GROUNDS FOR DENIAL | BASIS |
| 1.Integrated Application Form | -Check the consistency of applicant’s name, establishment’s name and address indicated in the Integrated Application Form
-Check the applicant’s signature |
-Non-submission
-Submitted integrated application form contains incomplete information |
FDA Circular No. 2014-003 |
| 2. Letter of Intent stating the specific changes | -It should state the specific changes made from the previously approved to proposed change/s. | It does not clearly state specific change/s made. Made change/s not included in the letter of intent. |
Republic Act 7394 |
| 3. Copy of initially approved Sales Promo Permit | -Clear and readable
-Should include all annexes attached
|
-Not readable | Administrative Order 4-A s, 1995 |
| 4. Promotional Materials, if any
|
-Clear and readable promotional materials
-The line “DOH-FDA-CFRR-Permit No. xxxx s. xxxx must be indicated |
-Not readable promotional materials
- The line “DOH-FDA-CFRR-Permit No. xxxx s. xxxx is not indicated. -With obvious health and nutritional claims and therapeutic claims e.g. Can cure diabetes Can lower blood cholesterol Superior amount of Vitamin C -With sexy tones, skin exposure, potentially controversial/sensitive execution -Use of the FDA Logo and Name - Non-inclusion of the Filipino Standard Message in prescribed format: “Mahalagang Paalala: Ang (name of product) ay hindi gamot at hindi dapat gamitin panggamot sa anumang uri ng sakit” for sales promotion application with Food Supplements as participating products |
Republic Act 7394
Bureau Circular No. 2007-002 FDA Memorandum Circular No. 2013-030 Administrative Order 2010-0008 |
| 5. Proof of Payment of Fees | -Payment details must be posted and verified by the FDA Cashier in the IFS-Document Tracking System | -Non-submission
- Payment details are not posted and verified by the FDA Cashier |
|
You may directly report your complaint to the manufacturer/ distributor of the product, if there are available consumer hotline services (telephone or mobile numbers) or email of the company. If not, you may download and fill out the FDA Product Complaint Form at https://www.fda.gov.ph/wp-content/uploads/2023/01/PCF-PRODUCT-COMPLAINT-FORM-2.pdf, attach the product picture in all angles, copy of receipts and all related supporting evidences / documents and send to the Food Safety Unit of CFRR at email address [email protected].
You may report it by downloading and filling-out the FDA Product Complaint Form at http://www.fda.gov.ph/advisories-2/others-advisories-pertaining-to-general-category/227368-fda-citizen-s-charter-handling-of-customer-complaints, attach the product picture in all angles, copy of receipts, and medical abstract from the hospital or clinic, medical certificate/ report from physician, and send to the Food Safety Unit of CFRR at email address [email protected]. If you are residing near FDA Alabang, you may bring and submit the sample to FDA. Otherwise, submit it to the nearest FDA accredited laboratory.
You may file your complaint at any nearest Acting Consumer Arbitration Office (ACAO) of the Department of Health (e.g. For Metro Manila residence, the ACAO is at DOH-Mandaluyong City).
You may send all the details of your report to [email protected].
Note: Your information will be treated with utmost confidentiality.
You may contact via their respective social media account or visit the nearest Local Government Unit in your area, specifically to the City Health Office or Sanitation Officer and file your complaint directly to them.
You may search the product on the website of FDA at fda.gov.ph or you can google the product then add FDA Advisory at the end
You may send us a letter requesting to lift the product and send it to our FDA Office or you may email it at [email protected] attaching the copy of CPR (Certificate of Product Registration), picture of the compliant product in commercial presentation, and the issued FDA Advisory for our evaluation.
To check if a food product or food supplement has been registered with the FDA, you may refer at https://verification.fda.gov.ph/
and search the name, brand or manufacturer/importer reflected in the label or the Food Registration (FR) Number of the product before purchasing
You may send your inquiries and/or clarifications with [email protected] . We will be happy to respond to your queries.
The SBs subject to excise tax are non-alcoholic beverages in liquid, powder or concentrates that are pre-packaged and sealed in accordance with FDA standards, that contain caloric sweetener and/or non-caloric sweeteners added by the manufacturers, and shall include, but not limited to the following, as described in the Food Category System from Codex Stan 192-1995 as adopted by the FDA:
Section 47 (A) Rate and Base of Tax (1) states that sweetened beverages using purely coconut sap sugar and purely steviol glycosides shall be exempt from tax.
Moreover, Section 47 (C) Exclusions indicates that the following beverages are exempted from taxation:
Ground coffee, instant soluble coffee and prepackaged powdered coffee products.
According to TRAIN Law, caloric sweeteners are substances that are sweet and includes sucrose, fructose, and glucose that produces a certain sweetness.
According to TRAIN Law, non- caloric sweeteners are substances that are artificially or chemically processed that produces a certain sweetness. These substances which can be directly added to beverages include aspartame, sucralose, saccharin, acesulfame potassium, neotame, cyclamates, and other non-nutritive sweeteners approved by the Codex Alimentarius and adopted by the FDA.
According to TRAIN Law, HFCS refers to a sweet saccharide mixture containing fructose and glucose which is derived from corn and added to provide sweetness to beverages, and which includes other similar syrup preparations.
According to PNS/BAFPS 76:2010, coconut sap sugar is a nutritive sweetener in solid form containing sucrose, glucose, and fructose, and derived from pure fresh coconut sap obtained by boiling.
According to FAO JECFA Monograph 23 (2019), steviol glycosides is a mixture of compounds extracted from the leaves of Stevia rebaudiana Bertoni containing a steviol backbone conjugated to any number or combination of the principal sugar moieties (glucose, rhamnose, xylose, fructose, arabinose, galactose and deoxyglucose) in any of the orientations occurring in the leaves of Stevia rebaudiana Bertoni. It is a white to light yellow powder, odourless or having slight characteristic odour, and about 200 to 300 times sweeter than sucrose.
Pre-packed beverage concentrates in liquid or powdered form to be sold to coffee or tea shops/restaurants/fast food operators/beverage manufacturers are exempted from excise tax if they are concentrates of any of the products enumerated under Section 47 (A) Rate and Base of Tax (1) and (C) Exclusions.
No. The FDA does not issue tax exemption certificate. It only provides responses to letter requests, stating the product categorization/classification according to Codex Stan 192-1995 and/or type of sweeteners used in the beverage product. The Bureau of Internal Revenue (BIR) or Bureau of Customs (BOC) determines whether a product is tax exempt or not, and/or how much tax is due based on the information on product classification according to Codex and/or type of sweeteners used provided by FDA.
No. Only those companies being required by the BIR/BOC to secure FDA confirmation of product classification according to Codex and/or type of sweeteners used should do so.
The results of laboratory tests mentioned is useful to determine the tax rate (Php 12 or 6 per Liter) of products covered by TRAIN Law. Thus, ideally, if a product is known to be tax exempt according to its Codex category, there is no need for it to be subjected to laboratory tests. However, for some reasons, products may sometimes be required by the BIR or BOC to obtain FDA confirmation of sweeteners used, which will need laboratory test results as supporting document in accordance with FDA Circular 2021-005.
The FDA Circular 2021-005 provides the Implementing Guidelines for Requesting FDA Confirmation of Product Classification and Type of Sweetener/s Used for SB Products.
DOST-FNRI, FAST Lab, SGS, and other FDA accredited laboratories capable of conducting sugar analysis and/or detecting non-caloric sweeteners.
All FDA responses are released at the FDA Action Center (FDAC), 3rd Floor, Starmall, Alabang. But a scanned copy may be e-mailed in advance to the requesting party upon notice.
Yes. According to Administrative Order (AO) No. 2014-0030-A, the type of sweetener/s used is a requirement covered by mandatory declaration of the complete list of ingredients including common name and functional category of food additives [e.g. refined sugar (sweetener)].
Yes. According to Administrative Order (AO) No. 2014-0030-A, the type of sweetener/s used is a requirement covered by mandatory declaration of the complete list of ingredients including common name and functional category of food additives [e.g. refined sugar (sweetener)].
According to AO No. 2014-0030-A, the labels of powdered beverages should indicate the number of liters per size or SKU, which shall be printed under the Direction/Instruction for Use (e.g. This pack makes 5 liters).
Coming soon…
Civic Drive Filinvest Corporate City
(02) 8857-1900
local 1000
(02) 8842-5635
Copyright © 2023. All Rights Reserved.