
Close

What is Pharmacovigilance?
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other medicine/vaccine related problem.
Before a medicine or vaccine is given approval by the FDA, the safety and efficacy of medicines are clinically proven through rigorous research with the aid of people who volunteer to take part in clinical trials. However, the process of clinical trials involves a relatively small number of volunteers for a short period of time. Other side effects, including rare side effects, are detected only when a medicine is used by a larger population with other concurrent diseases and over a longer period of time. Pharmacovigilance plays an important role in making sure that the medicines and vaccines we use are continuously providing benefits rather than the risk of having side effects.
No medicines or vaccines are proven 100% safe or no side effects at all. That is why it is important that the side effects experienced or identified are reported and evaluated. This will help us understand the problem and communicate effectively to prevent unnecessary harm. Everyone has a role to play to make our medicines and vaccines safe and effective for use.
Patients are encouraged to report the side effects that they experience from the use of medicines. Healthcare workers, including doctors, nurses, and pharmacists, are also encouraged to report side effects they encounter and those that are shared or reported by their patients. Market authorization holders (pharmaceutical companies who hold approval to market vaccines or medicinal products) are mandated to monitor and report side effects on the use of their products to the FDA.
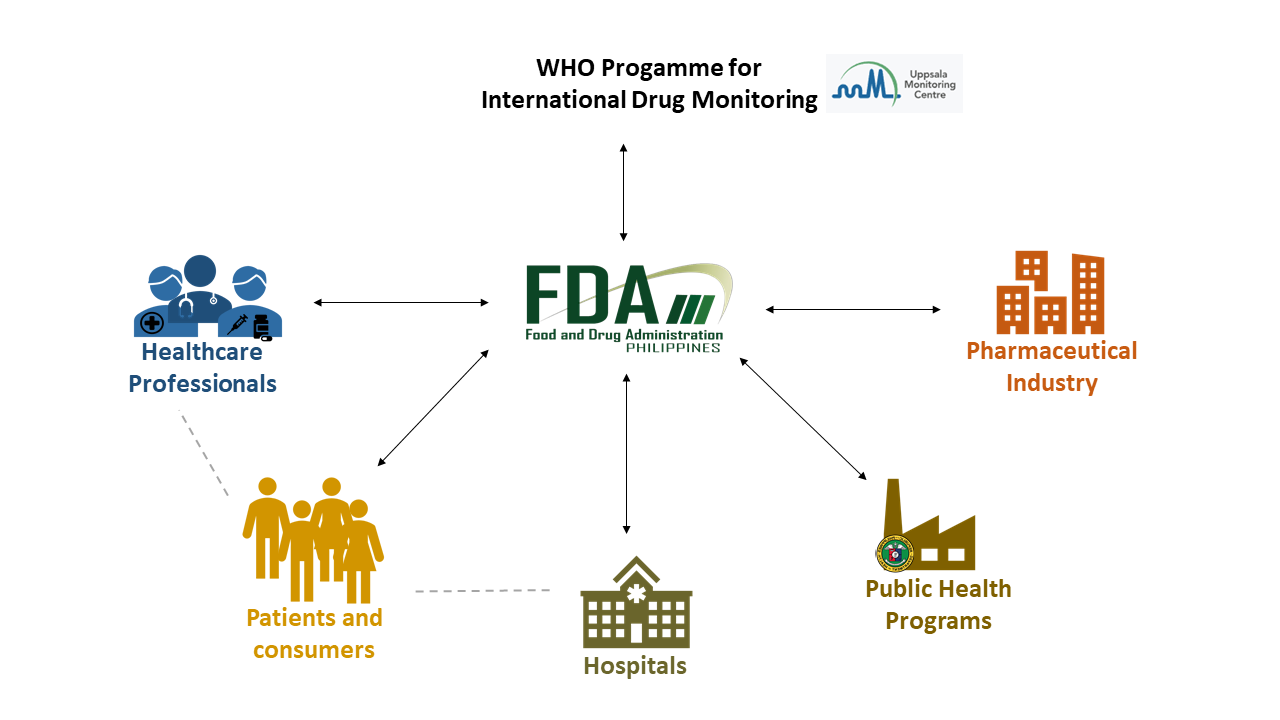
The FDA, through its Pharmacovigilance Section, is the National Pharmacovigilance Center in the country who receives and processes reports of suspected adverse drug reactions from pharmaceutical industries, hospitals, healthcare professionals, patients/consumers, and public health programs. The FDA works closely with the World Health Organization (WHO) Programme for International Drug Monitoring (PIDM).
The package insert or the patient information leaflet serves as the source of product information providing the list and information of adverse drug reactions that may be related to the use of a certain medicine. To better understand and provide more information on the safety of the products, any of the following should be reported:
Even if you are not sure if the medicine or vaccine caused the reaction, you may still report the suspected adverse reaction.
Adverse reactions are considered serious if they result in any of the following:
Patients
Patients or their caregiver/relatives are encouraged to report suspected side effects to medicines or vaccines. Click here for more information in reporting side effects.
Healthcare professionals
Doctors, nurses, and pharmacists are enjoined to report suspected adverse drug reactions experienced by their patients on the use of the products they prescribed, administered, or dispensed. Click here for more information for reporting adverse drug reactions.
Pharmaceutical industry
The pharmaceutical industry has the ultimate responsibility for the safety of their products. They are mandated to monitor the safety and efficacy of products approved for marketing by the FDA. To find more information on the pharmaceutical industry’s pharmacovigilance obligations, click here.
Reports from the patients and healthcare professionals are evaluated by the FDA. The primary reporter may receive the acknowledgement of the report or notification that the report has been successfully submitted. All valid reports are then entered into the national database. For reports with incomplete information, there will be a follow up to the reporter to complete the minimum information necessary for the evaluation.
Pharmaceutical industry submits the reports they collected to the FDA. The FDA, as an active member of the WHO Programme for International Drug Monitoring, forwards all the reports anonymously to the global database. The FDA Pharmacovigilance Team carefully screens all the reports for new risk. If a new risk is identified, the need for an action is evaluated and appropriate measures are taken into consideration.
Click here to access the WHO global database of reported potential side effects of medicinal products - the VigiAccess.
Every time you report a side effect, you are contributing to improve the safety of medicines and vaccines used by Filipinos.
Medicines and vaccines are registered to and evaluated by the FDA considering the quality of the products and current evidence of its safety and efficacy. However, no medicines are guaranteed 100% safe. Although they are carefully tested and evaluated, some side effects or adverse reactions may become evident only after the product is used by the general population.
Your report may contribute to:
Regulatory actions are imposed by the FDA to secure the safety of the public. It also provides consumers and healthcare professionals guidance on the rational use of medicines and vaccines.
Last updated: 11 August 2022
Civic Drive Filinvest Corporate City
(02) 8857-1900
local 1000
(02) 8842-5635
Copyright © 2023. All Rights Reserved.