FDA Advisory No. 2020-150-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Hindi Rehistradong Gamot na:
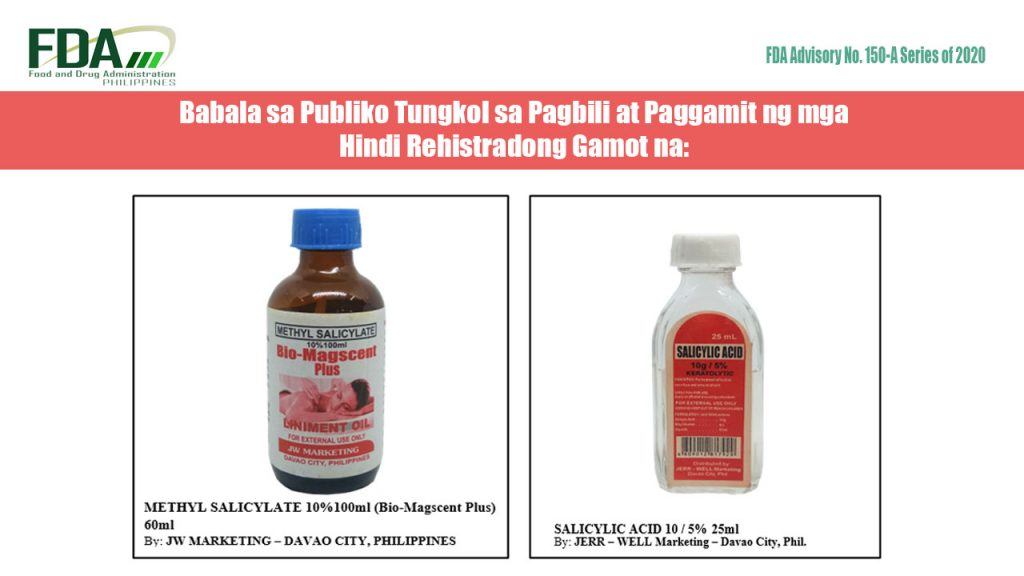
Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga hindi rehistradong gamot na: Methyl Salicylate 10%100ml (Bio-Magscent Plus) 60ml Salicylic Acid 10g / […]
FDA Advisory No. 2020-150 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:
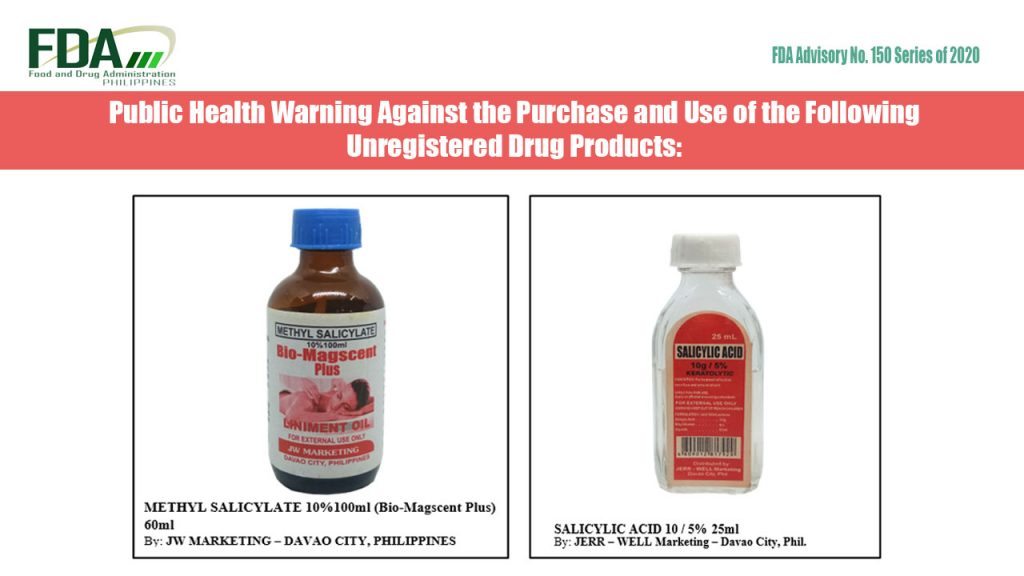
The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: Methyl Salicylate 10%100ml (Bio-Magscent Plus) 60ml Salicylic Acid 10g / […]
FDA Advisory No. 2020-149-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Hindi Rehistradong Gamot na Bayer Cyproterone acetate 2mg – Ethinylestradiol 0.035 mg (Diane®- 35) Tablet
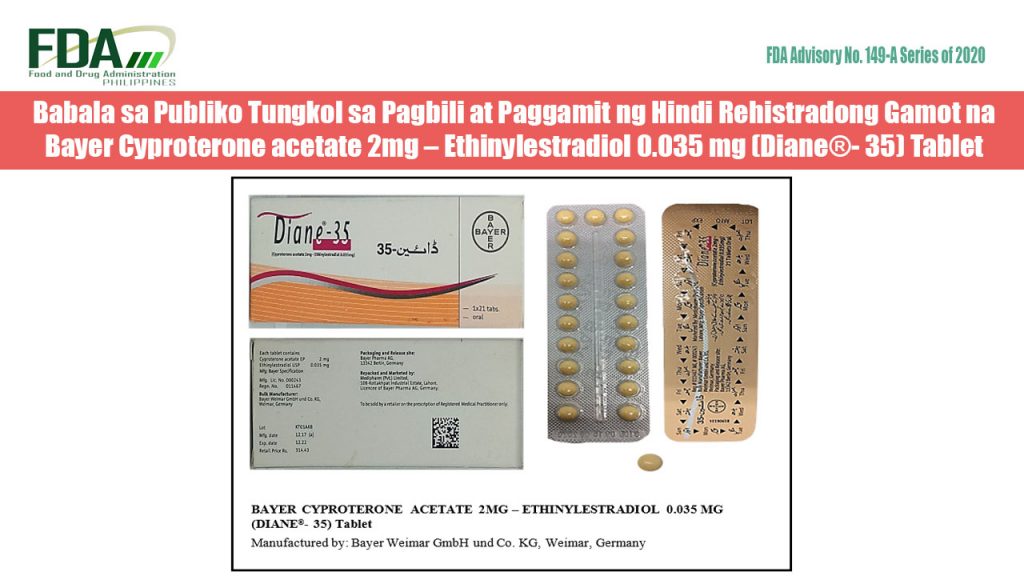
Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng hindi rehistradong gamot na: Bayer Cyproterone acetate 2mg – Ethinylestradiol 0.035 mg (Diane®- 35) Tablet […]
FDA Advisory No. 2020-149 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product Bayer Cyproterone acetate 2mg – Ethinylestradiol 0.035 mg (Diane®- 35) Tablet
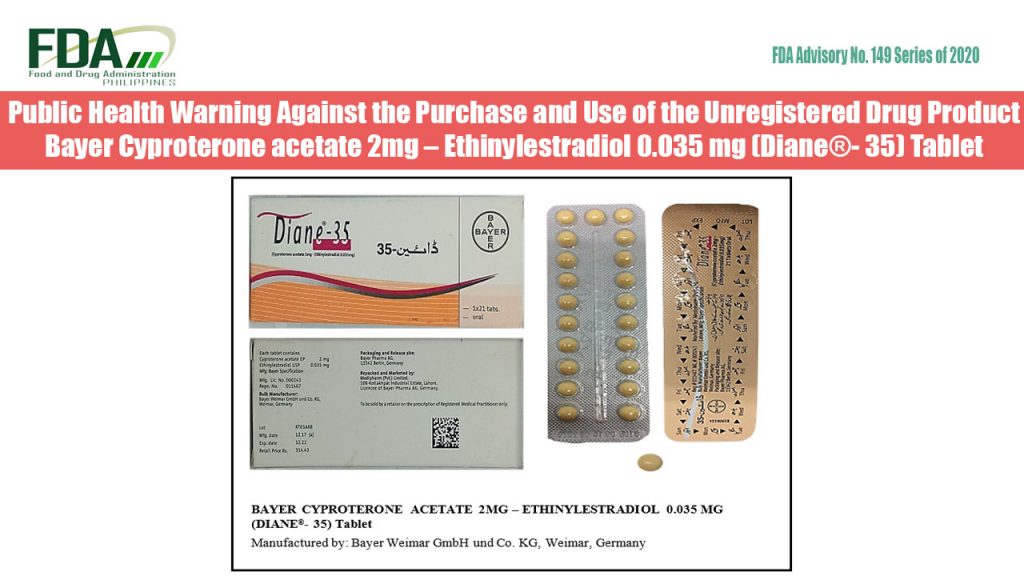
The Food and Drug Administration (FDA) advises the public against the purchase and use of the unregistered drug product: Bayer Cyproterone acetate 2mg – Ethinylestradiol 0.035 mg (Diane®- 35) Tablet […]
FDA Advisory No. 2020-131 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: DOLL INSTANT NOODLE CHEESE FLAVOUR MOOKIES BREAD & PASTRY SHOP SPICY BAGOONG […]
FDA Advisory No. 2020-130 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: ABC CRISPY PEANUT AND CASHEW JEF FLAVORED BANANA CHIPS – SOUR CREAM […]
FDA Advisory No. 2020-129 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: LUCKYSTAR SWEET COLA ROLLS MASTER SOFT ORANGE CANDY – ORANGE FILLING CHAM […]
FDA Advisory No. 2020-126 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: HILARIE FOOD PRODUCTS ROLL PASTILLAS (UNBRANDED) ROLL PASTILLAS HILARIE HOMEMADE STICK PASTILLAS […]
FDA Advisory No. 2020-115 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: GOOD TASTE CHAMPION PUDDING STICK JELLY SNS JELLY BEANS – MIX FLAVOURS […]
FDA Advisory No. 2020-114 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: LASAP WORCESTERSHIRE SAUCE ISLAND BISCUIT CHOKS CHOCOLATE COOKIE SUN BLOSSOM SPECIAL SAGO […]



