FDA Advisory No. 2020-1334 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food products: PREMIUM TABA NG TALANGKA CRAB PASTE MEMER TAMARIND […]
FDA Advisory No. 2020-1333 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Product and Food Supplements:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food product and food supplements: BLUE ECO FARM 100% RAW […]
FDA Advisory No. 2020-1332 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Product and Supplements:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food product and food supplements: FIGURES OF BEANS EUPHEMISM VANILLA BEANS NATROL KAVA KAVA […]
FDA Advisory No. 2020-1273 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consumption of the following unregistered food products: WIL-PACK Maple Flavoured Syrup ALMO Yabadoo Milk Monsters MARIANES Dried Pasas […]
FDA Advisory No. 2020-1272 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchase and consumption of the following unregistered food products: SUGARCANE LAND FOOD PRODUCT Piaya (Pinya) FARM & BARN Alkaline 12 […]
FDA Advisory No. 2020-805-A || Lifting the Advisory on the Registered Food Supplement GOLI NUTRITION APPLE CIDER VINEGAR GUMMIES DIETARY SUPPLEMENT under FDA Advisory No. 2020-805 Subject “Public Health Warning Against the Purchase and Consumption of the Unregistered Food Supplements”

The Food and Drug Administration (FDA) informs the public that the food supplement GOLI NUTRITION APPLE CIDER VINEGAR GUMMIES DIETARY SUPPLEMENT has been registered by the Market Authorization Holder, Alphacommerce Corporation in […]
Draft for Comments of Implementing Rules and Regulations on Revised Application Process and Requirements for PostApproval Changes of Pharmaceutical Products, and Institutionalization of the Philippine Variation Guidelines and Philippine Variation Guidelines V.1.0

All comments can be sent at [email protected]. Deadline of submission of comments shall be by 5 August 2020. Please see attachments: Attachment:->Draft Circular for Post Approval Changes and PVG Attachment:->Philippine Variation […]
FDA Advisory No. 2020-1373 || Public Health Warning Against the Purchase and Use of the Uncertified COVID-19 Test Kit “Wondfo® 2019-nCov in Foreign Characters”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public not to purchase and use of the uncertified COVID-19 test kit: “Wondfo® 2019-nCov in Foreign Characters” The […]
FDA Advisory No. 2020-1372 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Product “Filmed Laboratories Nanosoft™ Microneedles for Intradermal Injection”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product: “Filmed Laboratories Nanosoft™ Microneedles for Intradermal Injection” The […]
FDA Advisory No. 2020-1371 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Product “Hearty™ Sodium Hyaluronate Composite Solution for Injection in Foreign Characters”
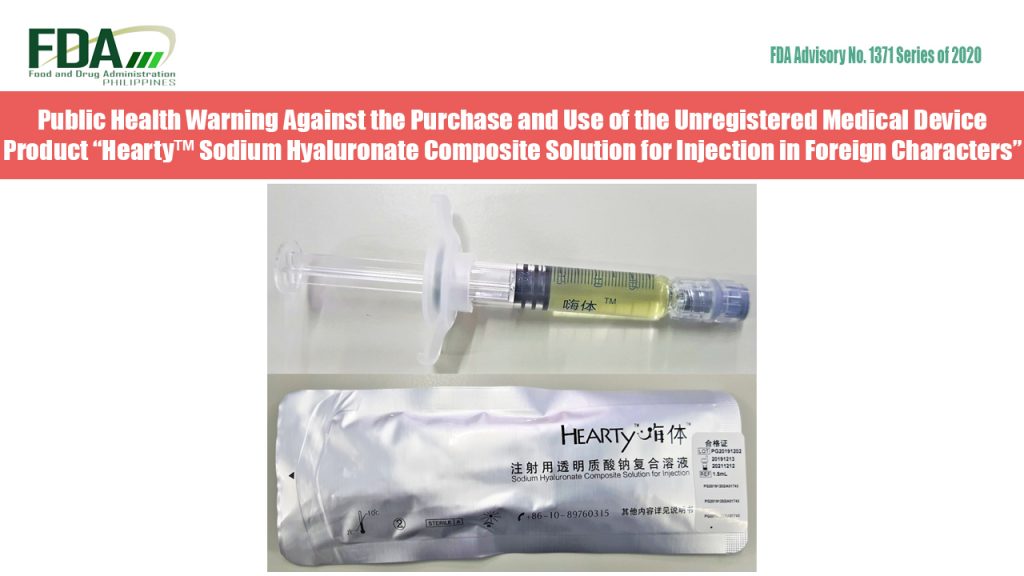
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product: “Hearty™ Sodium Hyaluronate Composite Solution for Injection in Foreign […]



