FDA Advisory No. 2020-054 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device “Whiter Smile Home Teeth Whitening Kit”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public against the purchase and use of the unregistered medical device: Whiter Smile Home Teeth Whitening Kit” […]
FDA Circular No. 2021-014 || Guidelines for the Use of the Food and Drug Administration (FDA) eServices Portal System for License to Operate (LTO) Application of Traders and Distributors including Wholesalers, Importers, and Exporters of Medical Devices, Equipment or Devices Used for Treating Sharps, Pathological and Infectious Waste and Water Treatment Devices/Systems

The objective of this Circular is to provide the guidelines on the FDA eServices Portal System in applying for LTO Application of Traders and Distributors including Wholesalers, Importers, and Exporters […]
FDA Circular No.2021-012 || Guidelines for the Use of the Food and Drug Administration (FDA) eServices Portal System for License to Operate (LTO) Application of Food Traders and Food Distributors Including Wholesalers, Importers, and Exporters of Processed Food Products, Food Supplements, Bottled Water, and Iodized Salt

I. BACKGROUND In order to build efficient regulatory systems, the Food and Drug Administration (FDA) has undertaken process improvement initiatives, including the establishment of information and communication technology infrastructures […]
Revised Bureau Circular No. 2007-010 entitled Guidelines in the Initial Issuance and Renewal of License to Operate for Iron Rice Premix Manufacturer / Repacker / Importer and Updating of the Standards for Iron Rice Premix

OBJECTIVES: This Circular aims to provide updated guidelines and standards on the suitable level of iron in rice in the manufacture of iron-rice premix to help address iron deficiency anemia […]
FDA Circular No. 2021-008 || Updated Guidelines for the Registration of Drug Products under Emergency Use (DEU) for COVID-19
OBJECTIVE: This Circular aims to provide streamlined requirements and application process for the registration of Drug Products for COVID-19. Attachments FDA Circular No. 2021-008 (2 MB)
Clarification on the Approval and Use of Leronlimab

The Food and Drug Administration (FDA) clarifies that Leronlimab is not approved by the FDA for treatment of COVID-19. Leronlimab is an investigational product which is still undergoing clinical trials […]
FDA Advisory No.2021-0269-A || Lifting the Advisory on the Registered Food Product KIK’S Tamarind Sweet Chili Flavor “Public Health Warning Against the Purchase and Consumption of the Unregistered Food Products”

The Food and Drug Administration (FDA) informs the public that the food product KIK’S Tamarind Sweet Chili Flavor has been registered by the Market Authorization Holder (MAH) E.C. Sebuala Marketing […]
FDA Advisory No.2021-0525 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Products in foreign characters

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the following unregistered medical device products: The FDA verified through post-marketing […]
FDA Advisory No.2021-0524 || Public Health Warning Against the Purchase and Use of Counterfeit Medical Device Product “Mediclean Face Mask”
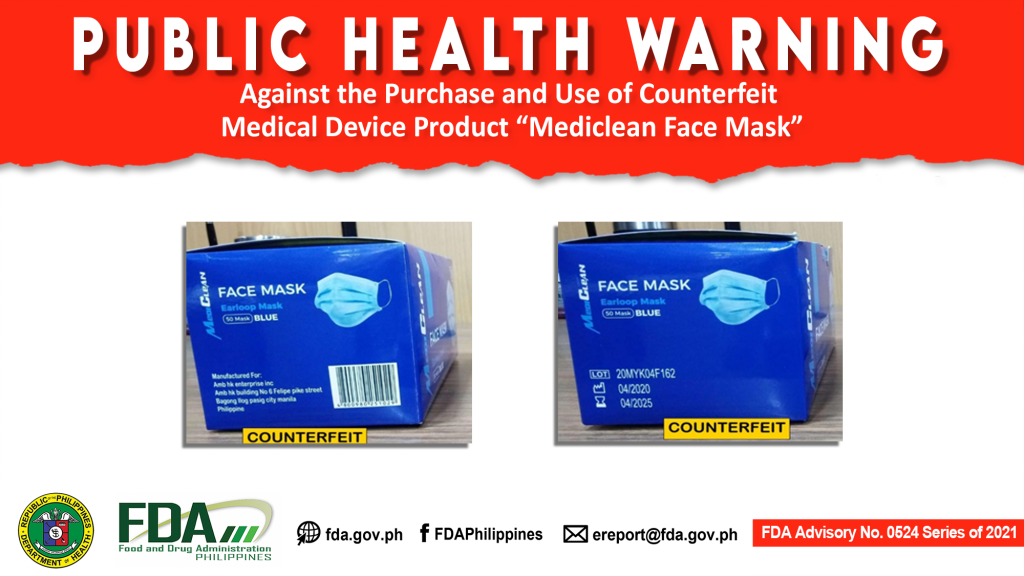
The Food and Drug Administration (FDA) advises the public from purchasing and using the counterfeit medical device: The FDA has coordinated with the Market Authorization Holder (MAH), AMB HK Enterprises […]
FDA Advisory No.2020-0665-A || Lifting the Advisory on the Notified Cosmetic Product “COLOURETTE COLOURSNAPS MILKY” under FDA Advisory No. 2020-665 “Public Health Warning Against the Purchase and Use of Unnotified Cosmetic COLOURETTE MILK COLOURSNAP DEWY MULTI-STAIN” Dated 15 April 2020

The Food and Drug Administration (FDA) informs the public that the Cosmetic product Colourette Coloursnaps Milky with 1000007447496, has been notified by the Market Authorization Holder, NIV DELLA BEAUTY INNOVATIONS […]



