FDA Advisory No.2024-0669 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “DEOPROCE SKIN RESCUE LIGHTWEIGHT SUNSCREEN SPF50 PA+++ 06”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, DEOPROCE SKIN RESCUE LIGHTWEIGHT SUNSCREEN SPF50 PA+++ 06. The abovementioned product was verified […]
FDA Advisory No.2024-0668 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SANTA MARIA VIRGIN COCONUT OIL ALOE VERA”
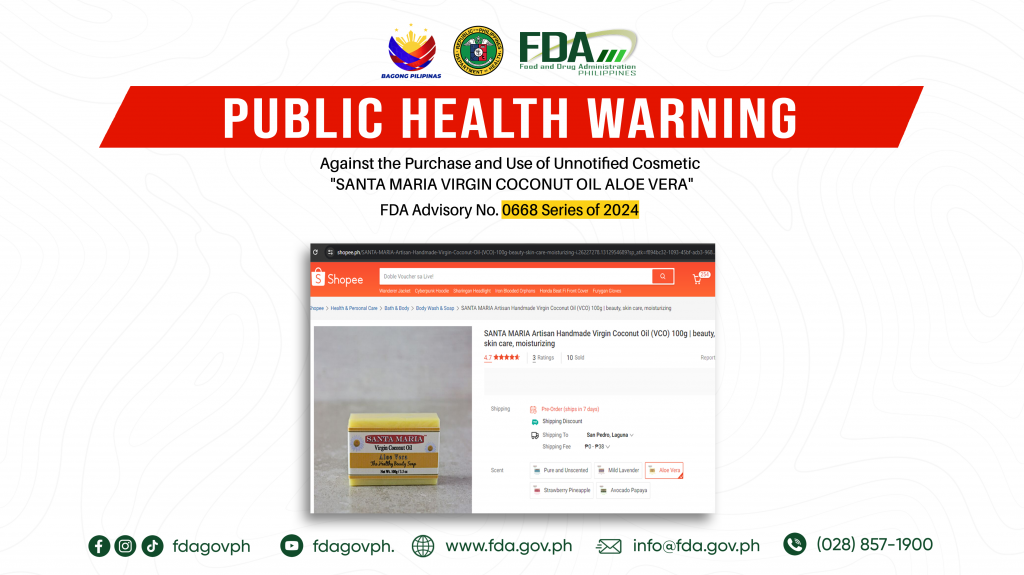
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SANTA MARIA VIRGIN COCONUT OIL ALOE VERA. The abovementioned product was verified by […]
FDA Advisory No.2024-0667 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SOUG WELL HAIR REPAIR AND MAINTENANCE HAIR MASK”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SOUG WELL HAIR REPAIR AND MAINTENANCE HAIR MASK. The abovementioned product was verified […]
FDA Advisory No.2024-0666 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SY GLOW DEO PROTECT TONER”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SY GLOW DEO PROTECT TONER. The abovementioned product was verified by FDA through […]
FDA Advisory No.2024-0665 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SY GLOW WHITENING BEAUTY DEO INSTANT WHITE PROTECT CREAM”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SY GLOW WHITENING BEAUTY DEO INSTANT WHITE PROTECT CREAM. The abovementioned product was […]
FDA Advisory No.2024-0664 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SANTA MARIA VIRGIN COCONUT OIL – MILD”
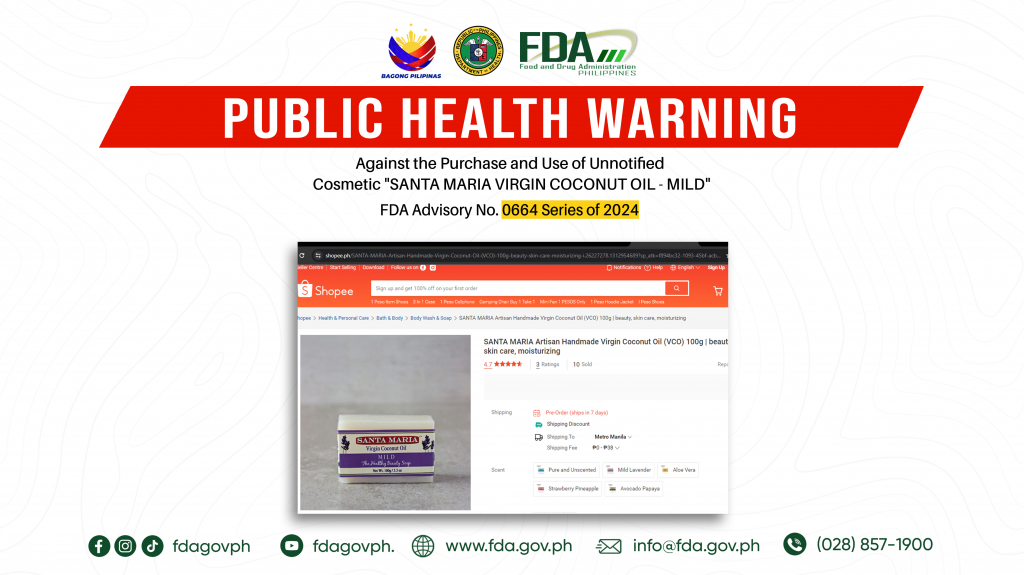
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SANTA MARIA VIRGIN COCONUT OIL – MILD. The abovementioned product was verified by […]
FDA Advisory No.2024-0663 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SUNISA ORANGE EXFOLIATING WHITENING GEL”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SUNISA ORANGE EXFOLIATING WHITENING GEL. The abovementioned product was verified by FDA through […]
FDA Advisory No.2024-0662 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “KLARICO SUPER HAIR NATURAL ANTI-HAIRFALL AND THICKENING SHAMPOO”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, KLARICO SUPER HAIR NATURAL ANTI-HAIRFALL AND THICKENING SHAMPOO. The abovementioned product was verified […]
FDA Advisory No.2024-0670 || REMINDERS ON THE SAFE USE OF SWINGS, SLIDES, AND SIMILAR ACTIVITY TOYS FOR INDOOR AND OUTDOOR FAMILY DOMESTIC USE
Summer is the perfect time for kids to stay active and play with swings, slides, and activity toys, transforming any room or backyard into a playground of excitement and laughter. […]
FDA Advisory No.2024-0627 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SYMMETRY LAB. PARFUMS SANDALWOOD SEX EXTRAIT DE PARFUM”
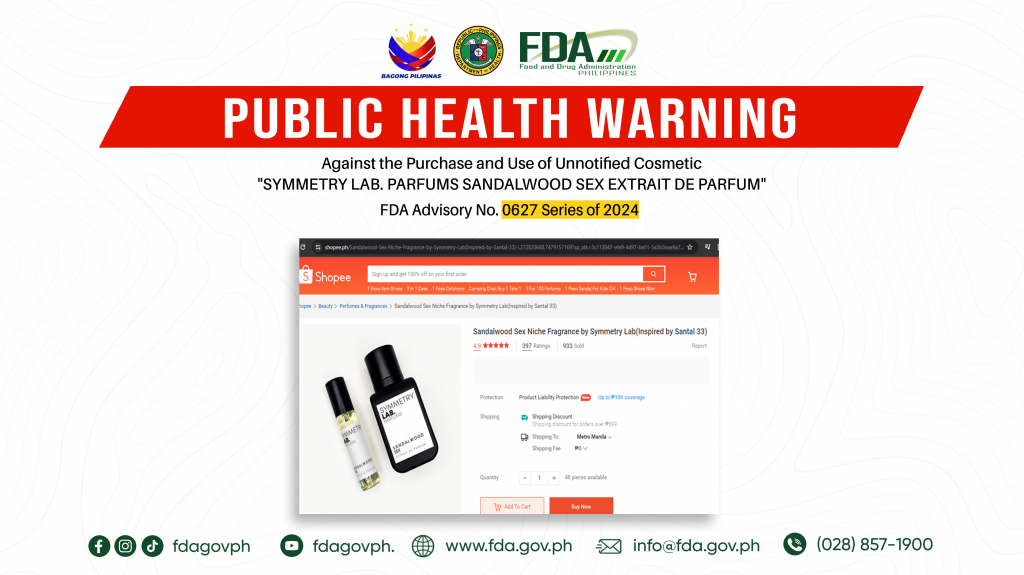
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SYMMETRY LAB. PARFUMS SANDALWOOD SEX EXTRAIT DE PARFUM. The abovementioned product was verified […]



