FDA Advisory No.2024-0649 || Public Health Warning Against the Purchase and Consumption of the Unregistered Food Supplement “SPROUT Magnesium Glycinate Dietary Supplement”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the unregistered food supplement: 1. SPROUT Magnesium Glycinate Dietary Supplement The […]
FDA Advisory No.2024-0644 || Public Health Warning Against the Purchase and Consumption of the Unregistered Food Supplement “KAPENSTEND Ginkgo Biloba Herbal Supplements Dietary Supplement”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND CONSUME the following unregistered food supplement: 1. KAPENSTEND Ginkgo Biloba Herbal Supplements […]
FDA Advisory No.2024-0636 || Public Health Warning Against the Purchase and Use of Unregistered Household/Urban Pesticide (HUP) “KJ INSECTICIDAL AEROSOL (ROSE FLAVOR)”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unregistered household/urban pesticide (HUP) product, KJ INSECTICIDAL AEROSOL (ROSE FLAVOR). The abovementioned product was verified by […]
FDA Advisory No.2024-0635 || Public Health Warning Against the Purchase and Use of Unregistered Household/Urban Pesticide (HUP) “GUIWANG FROGKING INSECTICIDE AEROSOL JASMINE”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unregistered household/urban pesticide (HUP) product, GUIWANG FROGKING INSECTICIDE AEROSOL JASMINE. The abovementioned product was verified by […]
FDA Advisory No.2024-0634 || Public Health Warning Against the Purchase and Use of Unregistered Household/Urban Pesticide (HUP) “WAWANG FROGKING AEROSOL INSECTICIDE JASMINE ESSENCE”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unregistered household/urban pesticide (HUP) product, WAWANG FROGKING AEROSOL INSECTICIDE JASMINE ESSENCE. The abovementioned product was verified […]
FDA Advisory No.2024-0633 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SKIN HALO SUNSCREEN GLOW SPF 50+++”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SKIN HALO SUNSCREEN GLOW SPF 50+++. The abovementioned product was verified by FDA […]
FDA Advisory No.2024-0632 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SANTA MARIA VIRGIN COCONUT OIL PURE & UNSCENTED”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SANTA MARIA VIRGIN COCONUT OIL PURE & UNSCENTED. The abovementioned product was verified […]
FDA Advisory No.2024-0631 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SANTA MARIA VIRGIN COCONUT OIL PINEAPPLE STRAWBERRY”
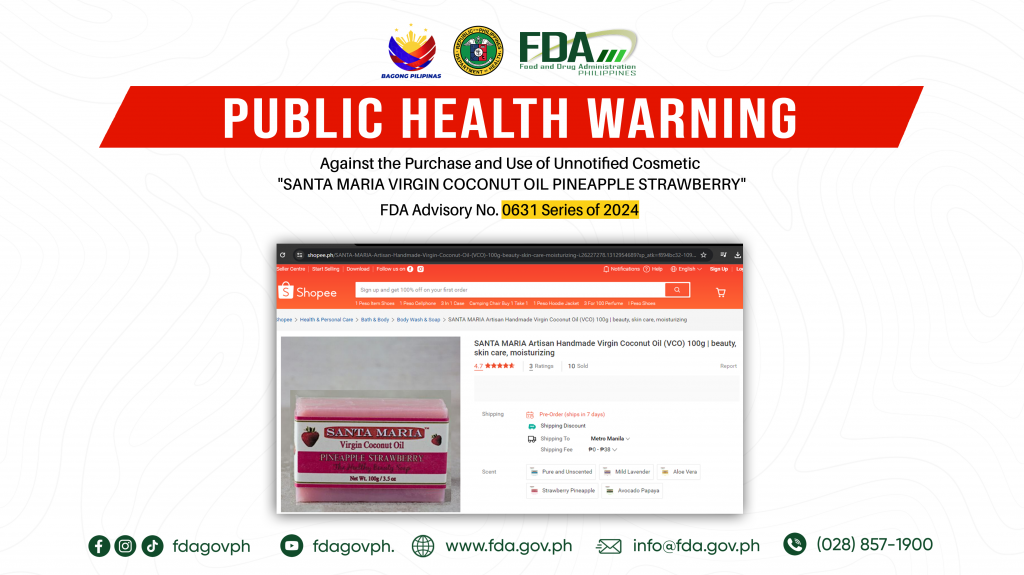
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SANTA MARIA VIRGIN COCONUT OIL PINEAPPLE STRAWBERRY. The abovementioned product was verified by […]
FDA Advisory No.2024-0630 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “SANTA MARIA VIRGIN COCONUT OIL AROMATHERAPY BODY MASSAGE OIL”
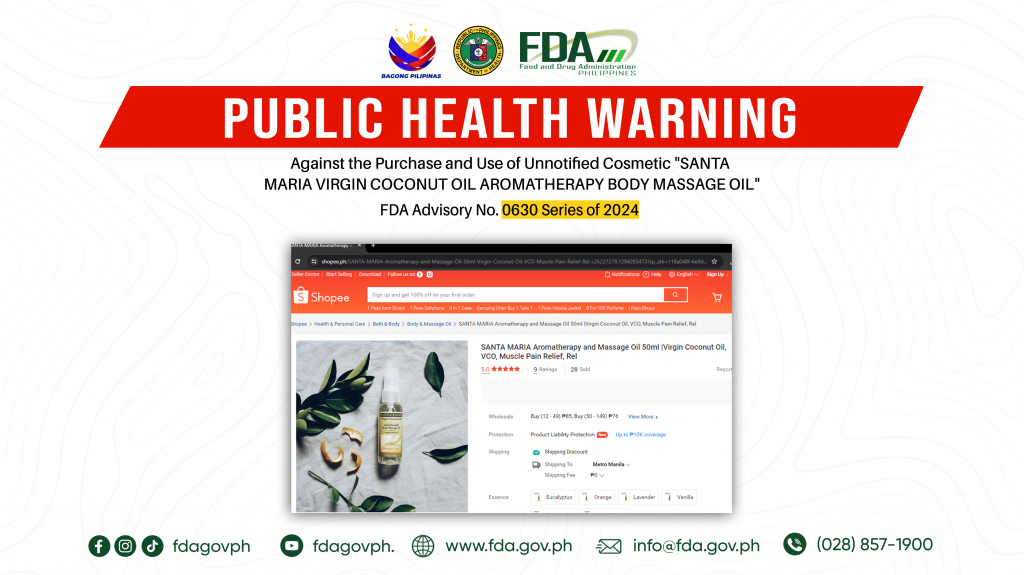
The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, SANTA MARIA VIRGIN COCONUT OIL AROMATHERAPY BODY MASSAGE OIL. The abovementioned product was […]
FDA Advisory No.2024-0379 || Public Health Warning Against the Purchase and Use of Unnotified Cosmetic “BELLA AMORE SKIN HIMALAYAN HEALING SOAP”

The Food and Drug Administration (FDA) warns the public NOT TO PURCHASE AND USE unnotified cosmetic product, BELLA AMORE SKIN HIMALAYAN HEALING SOAP. The abovementioned product was verified by FDA […]



