The Food and Drug Administration (FDA) advises the public against the purchase and use of the counterfeit version of the following products:
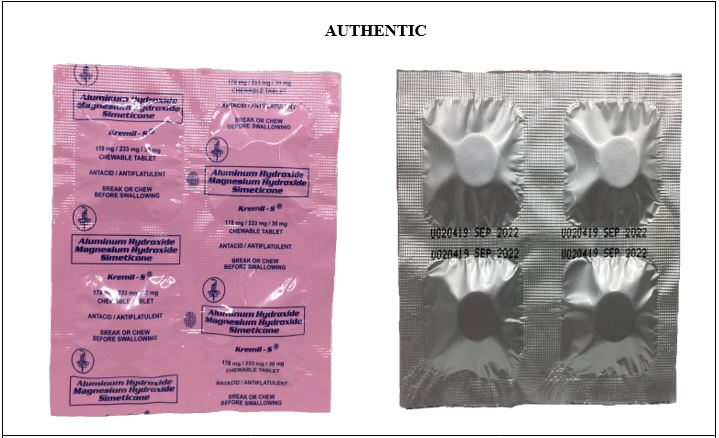
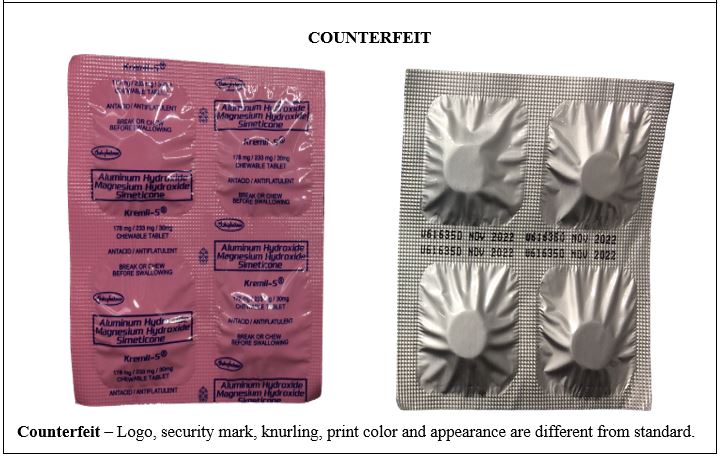
Figure 1. Comparison between the Authentic and Counterfeit Aluminum Hydroxide / Magnesium Hydroxide / Simeticone (Kremil-S®) 178mg/233mg/30mg Chewable Tablet (Lot Nos. U213457 and U616350)
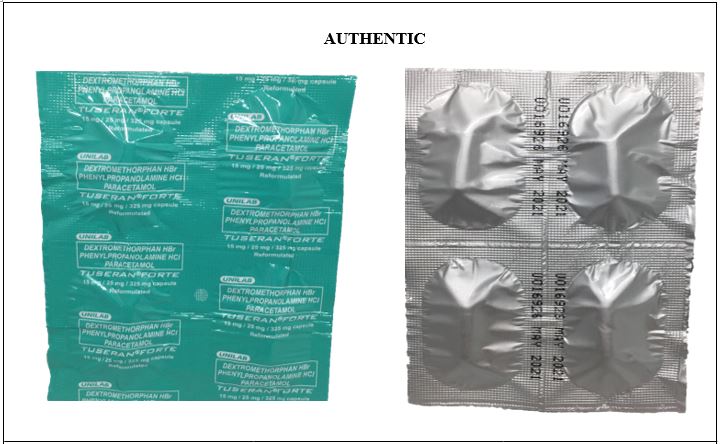

Figure 2. Comparison between the Authentic and Counterfeit Dextromethorphan HBr Phenylpropanolamine HCl / Paracetamol (Tuseran® Forte) 15mg/25mg/325 mg Capsule (Lot Nos. U009818 and U013768)
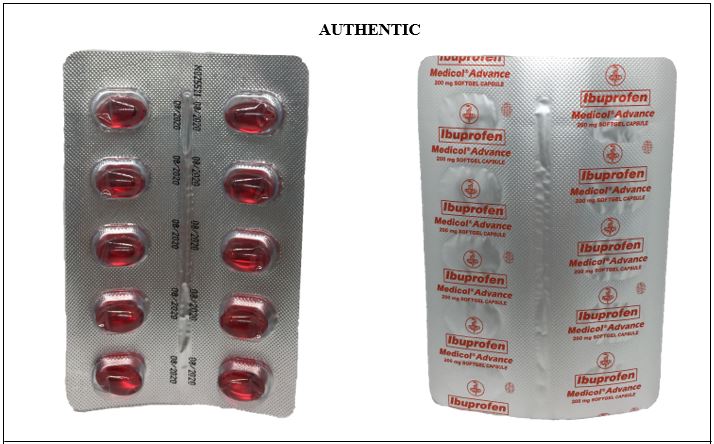
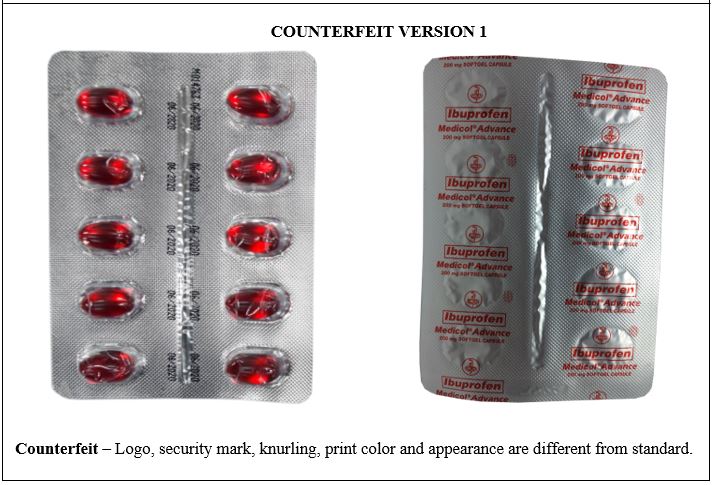 Figure 3-A. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
Figure 3-A. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
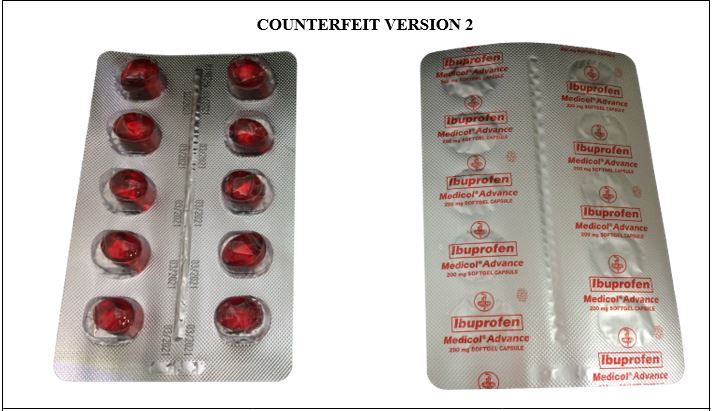
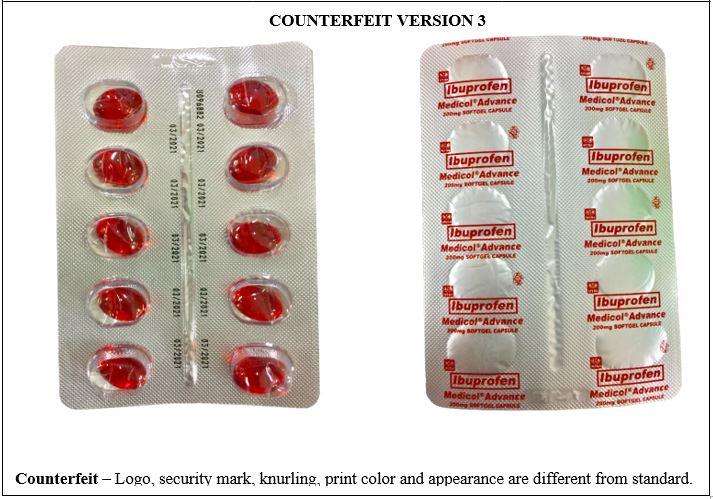
Figure 3-B. Comparison between the Authentic and Counterfeit Ibuprofen (Medicol® Advance) 200mg Soft Gelatin Capsule (Lot nos. M019221, M014763, M011806, and U096882)
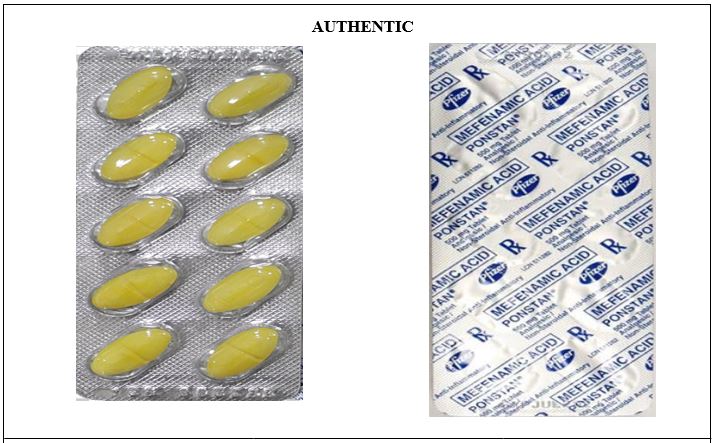
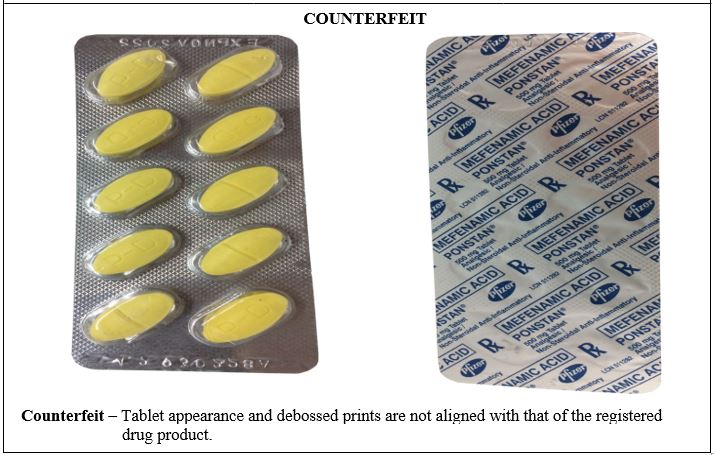
Figure 4. Comparison between the Authentic and Counterfeit Mefenamic Acid (Ponstan®) 500mg Tablet (Lot nos. 930228A and 42930228A)
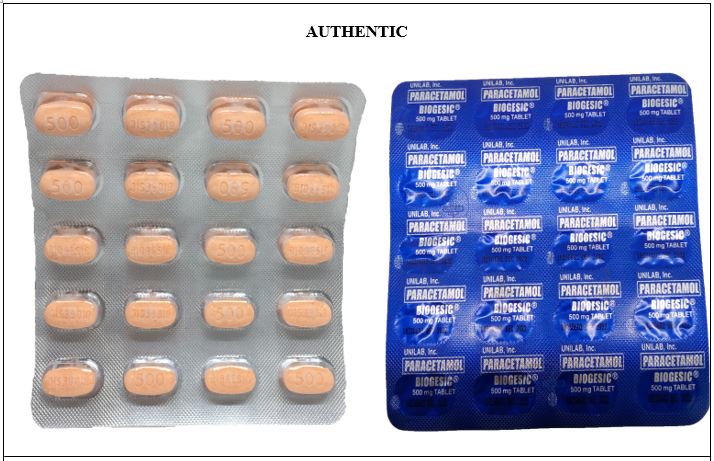
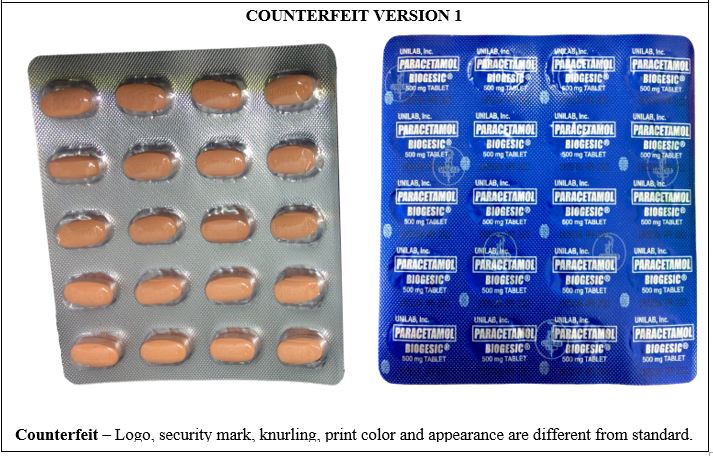
Figure 5-A. Comparison between the Authentic and Counterfeit Paracetamol (Biogesic®) 500mg Tablet (Lot nos. 19047723, 16885778, and 19052306)
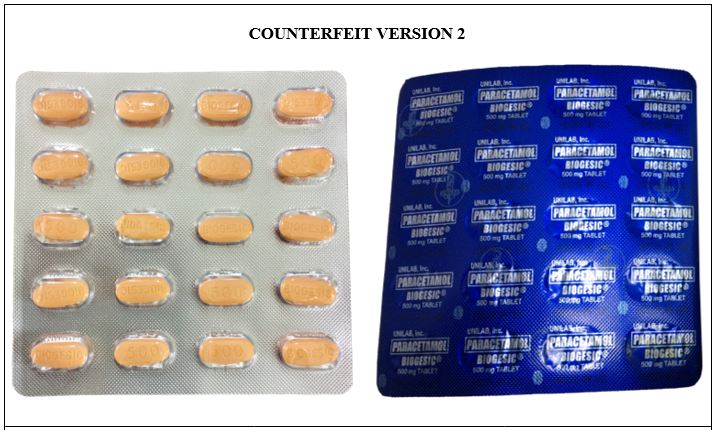
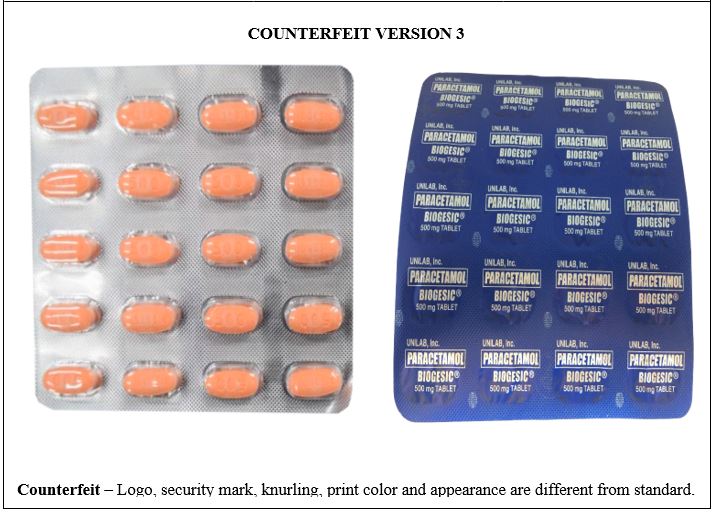
Figure 5-B. Comparison between the Authentic and Counterfeit Paracetamol (Biogesic®) 500mg Tablet (Lot nos. 19047723, 16885778, and 19052306)
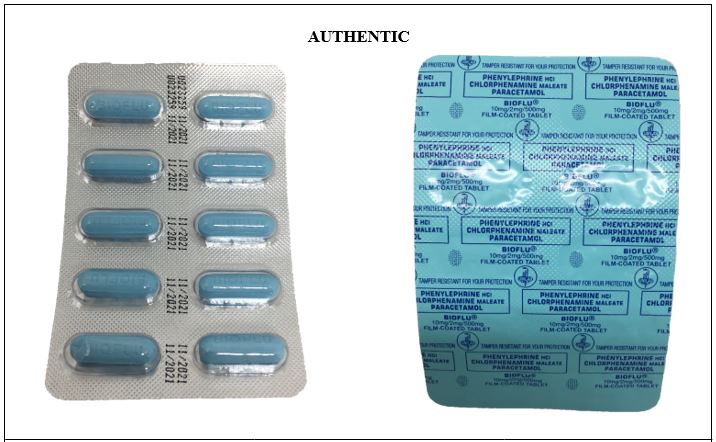
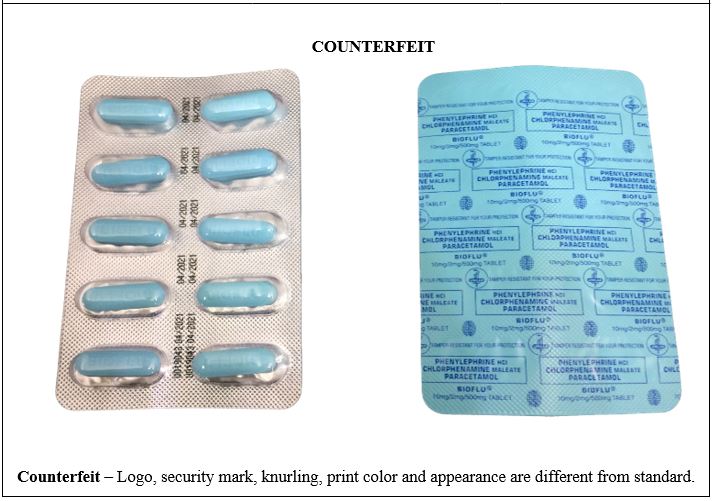
Figure 6. Comparison between the Authentic and Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Bioflu®) 10mg/2mg/500mg Tablet (Lot nos. U019043, 0190415, and 19052306)
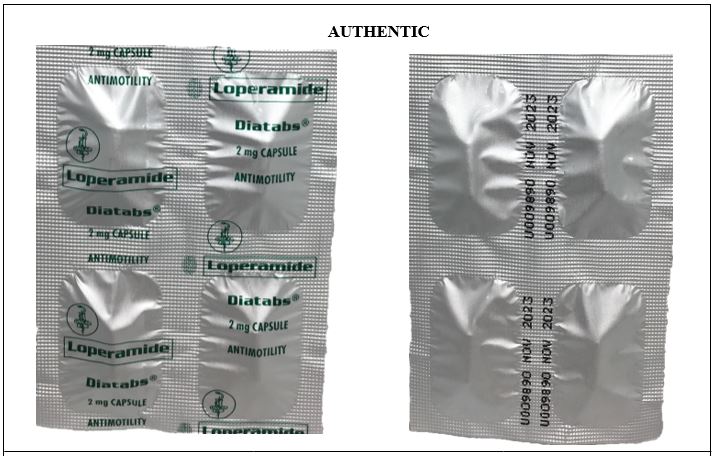
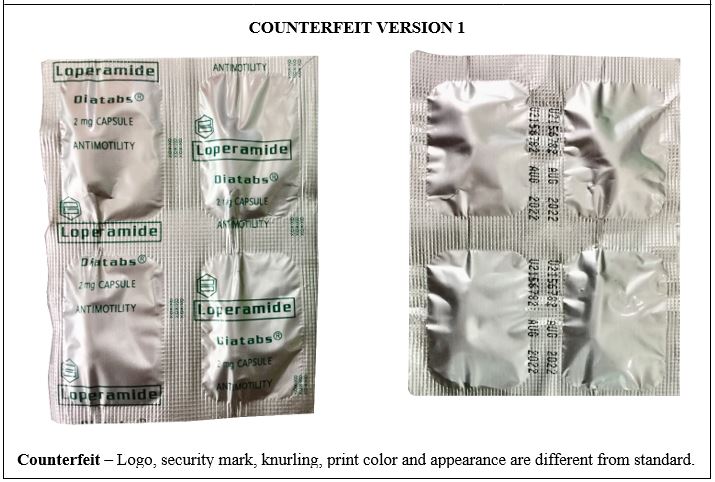
Figure 7-B. Comparison between the Authentic and Counterfeit Loperamide (Diatabs®) 2mg Capsule (Lot nos. U2156782, U455238, and U3167321)
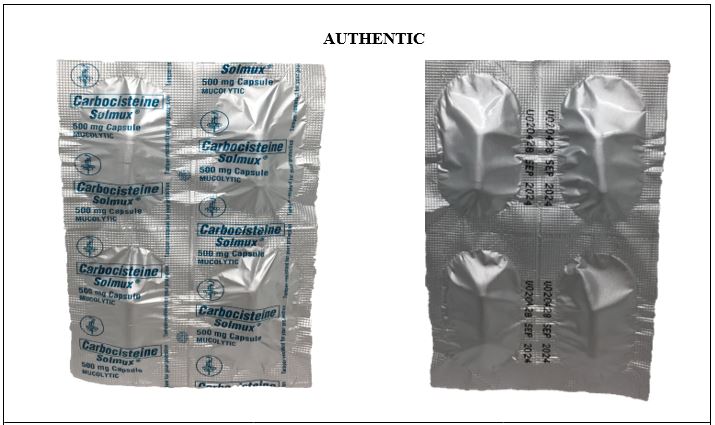
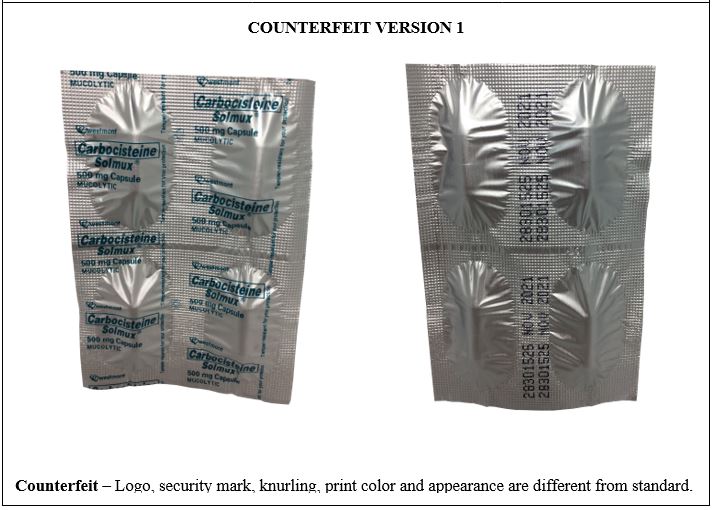
Figure 8-A. Comparison between the Authentic and Counterfeit Carbocisteine (Solmux®) 500mg Capsule (Lot nos. 28301525, U009159, and 28301525)

Figure 8-B. Comparison between the Authentic and Counterfeit Carbocisteine (Solmux®) 500mg Capsule (Lot nos. 28301525, U009159, and 28301525)
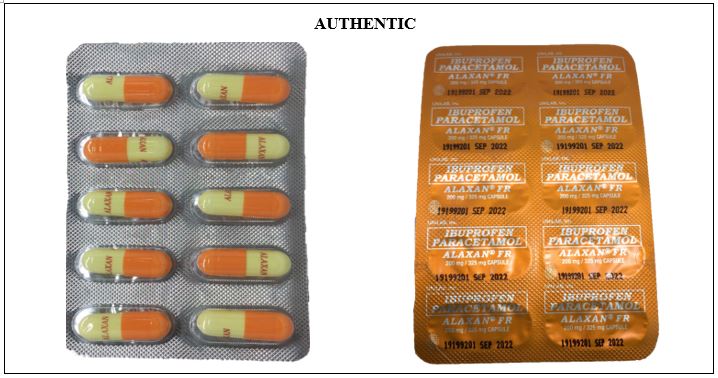
Figure 9-A. Comparison between the Authentic and Counterfeit Ibuprofen / Paracetamol (ALAXAN®)FR 200mg/325mg Capsule (Lot nos. 18090711, 18090705, 19070126, 19030110, 19090828 and 18101901)
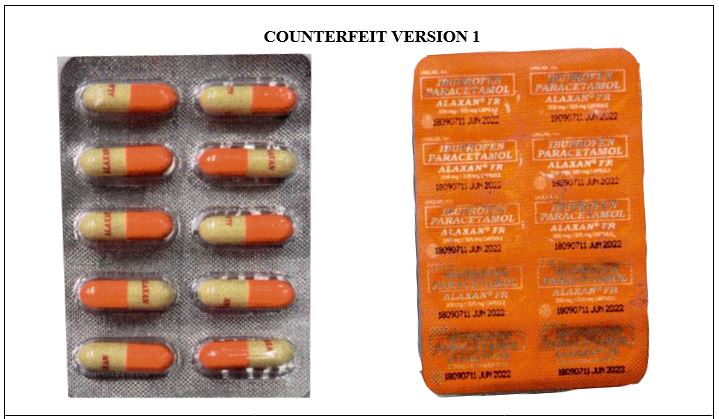
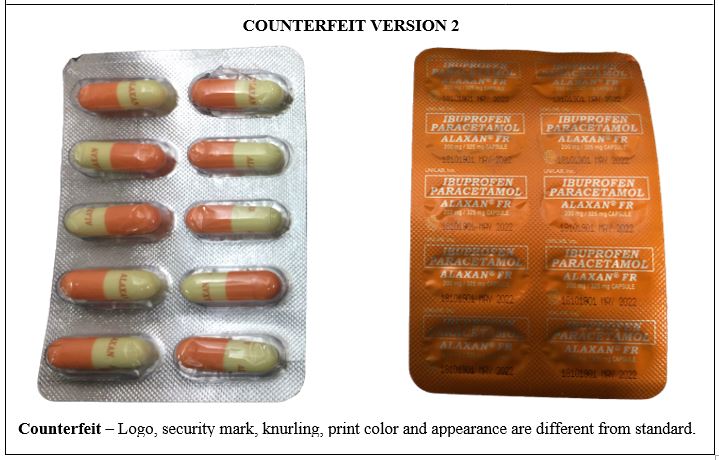
Figure 9-B. Comparison between the Authentic and Counterfeit Ibuprofen / Paracetamol (ALAXAN®) FR 200mg/325mg Capsule (Lot nos. 18090711, 18090705, 19070126, 19030110, 19090828 and 18101901)
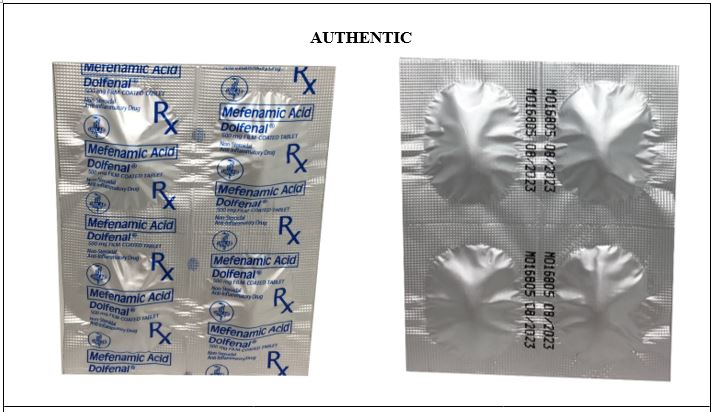
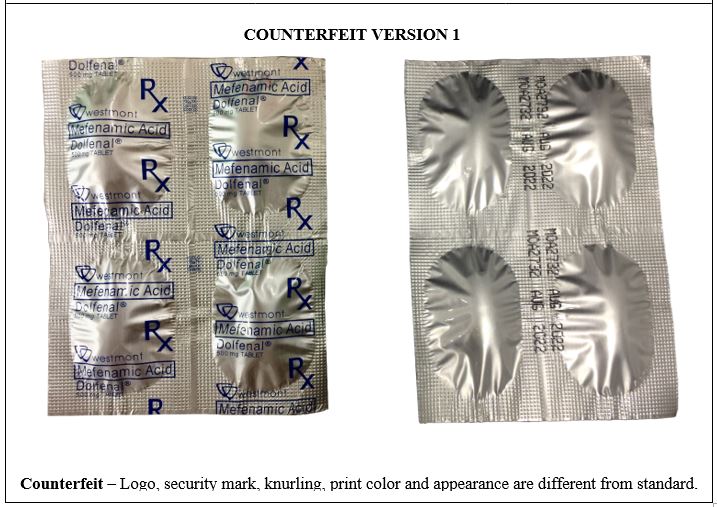
Figure 10-A. Comparison between the Authentic and Counterfeit Mefenamic Acid (Dolfenal®) 500mg Tablet (Lot nos. MOA2732 and MOA1705)
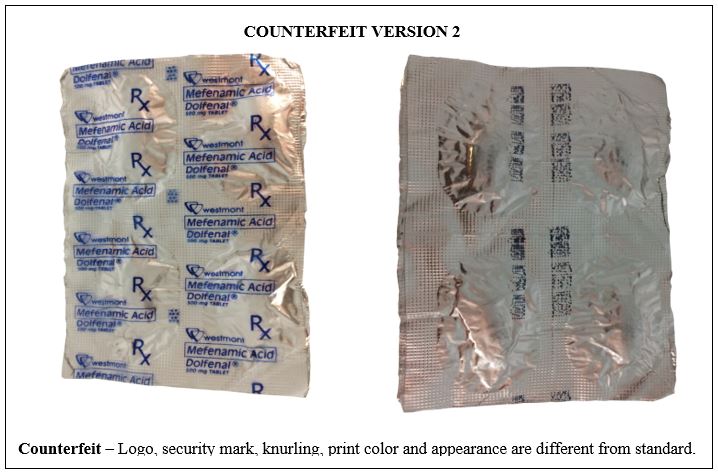
Figure 10-B. Comparison between the Authentic and Counterfeit Mefenamic Acid (Dolfenal®) 500mg Tablet (Lot nos. MOA2732 and MOA1705)
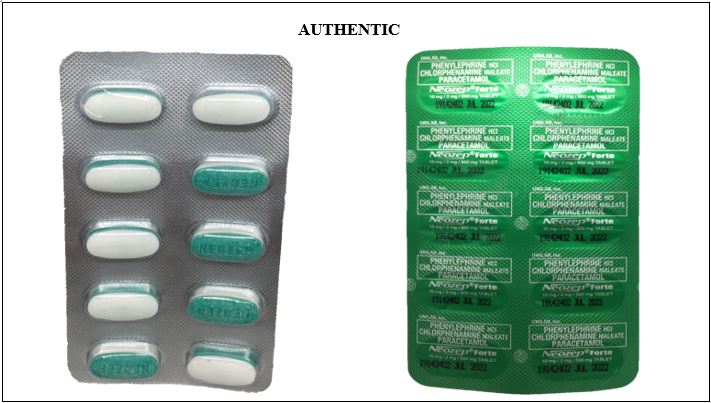
Figure 11-A. Comparison between the Authentic and Verified Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10 mg/2 mg/500 mg Tablet (Lot nos. 19067382, 18212086, and 18012006)
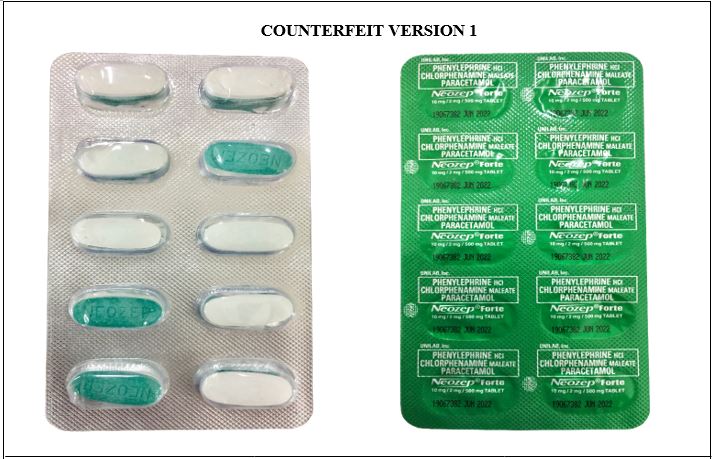
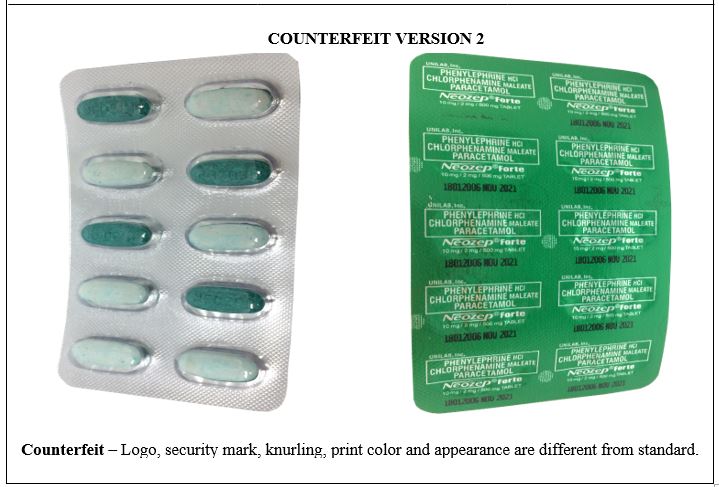
Figure 11-B. Comparison between the Authentic and Verified Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10 mg/2 mg/500 mg Tablet (Lot nos. 19067382, 18212086, and 18012006)
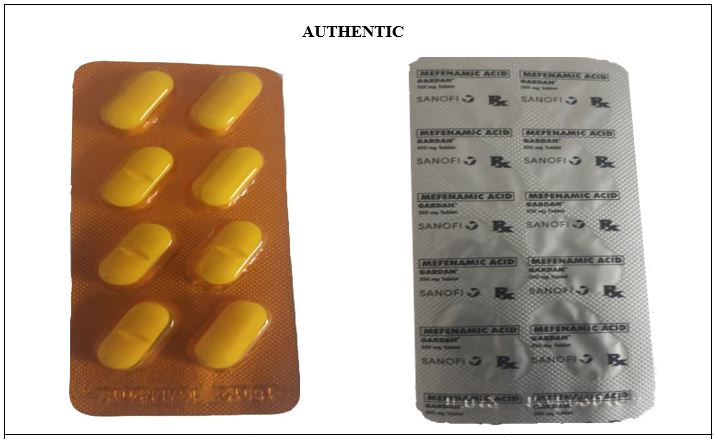
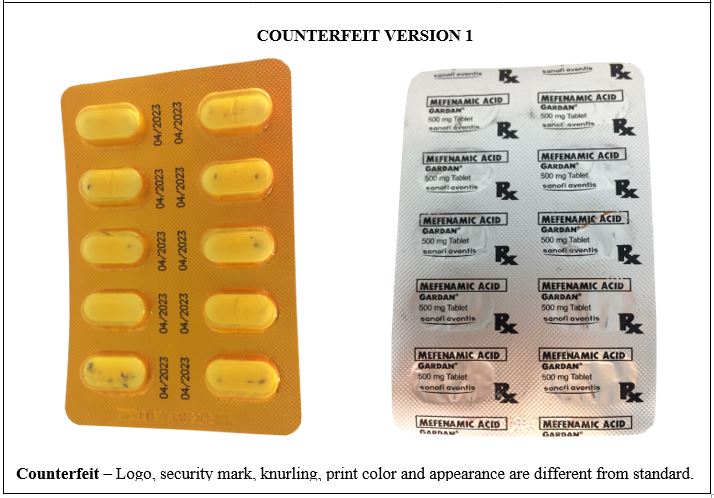
Figure 12-A. Comparison between the Authentic and Counterfeit Mefenamic Acid (Gardan®) 500mg Tablet (Lot nos. 19143 and 19030)
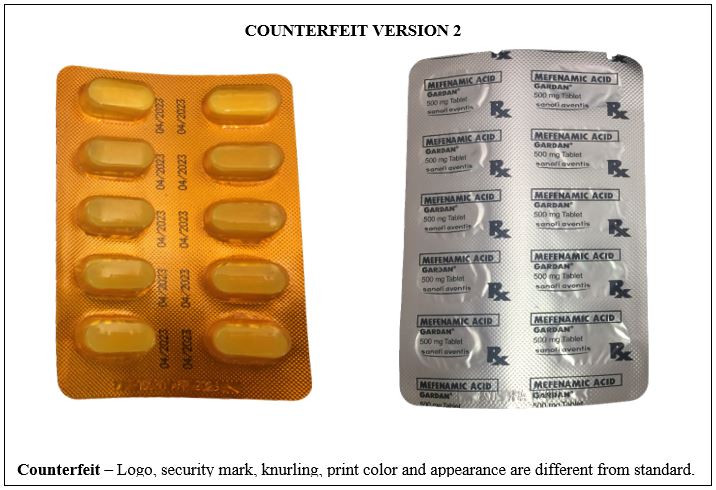
Figure 12-B. Comparison between the Authentic and Verified Counterfeit Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10 mg/2 mg/500 mg Tablet (Lot nos. 19067382, 18212086, and 18012006)
All healthcare professionals and the general public are hereby warned as to the availability of these counterfeit drug products in the market which pose potential danger or injury to consumers. Consumers are also reminded to purchase drug products only from FDA-licensed establishments.
Likewise, all establishments and outlets are hereby warned against selling and/or dispensing of the said counterfeit products with the abovementioned features. The importation, selling or offering for sale of such is in direct violation of Republic Act No. 9711 or the Food and Drug Administration Act of 2009, and Republic Act No. 8203 or the Special Law on Counterfeit Drugs. Anyone found selling the said counterfeit drug products will be penalized.
All Local Government Units (LGUs) and Law Enforcement Agencies (LEAs) are requested to ensure that these products are not sold or made available in their localities or areas of jurisdiction.
For more information and inquiries, please e-mail us at [email protected]. To report continuous sale or distribution of unregistered health products, kindly e-mail us via [email protected], or through the online reporting facility, eReport, at www.fda.gov.ph/ereport. You may also call the Center for Drug Regulation and Research at telephone number (02) 8809-5596. For any suspected adverse drug reaction (ADR), report immediately to FDA through this link: https://primaryreporting.who-umc.org/Reporting/Reporter?OrganizationID=PH and fill out all the required fields.
Dissemination of the information to all concerned is requested.
Attachment:->FDA Advisory No.2020-1348



