The Food and Drug Administration (FDA) notifies the public on the WHO Medical Product Alert on a substandard (contaminated) Methotrexate with brand name “Methotrex” which were detected in Eastern Mediterranean region:
- Methotrex” Confirmed by the World Health Organization (WHO)
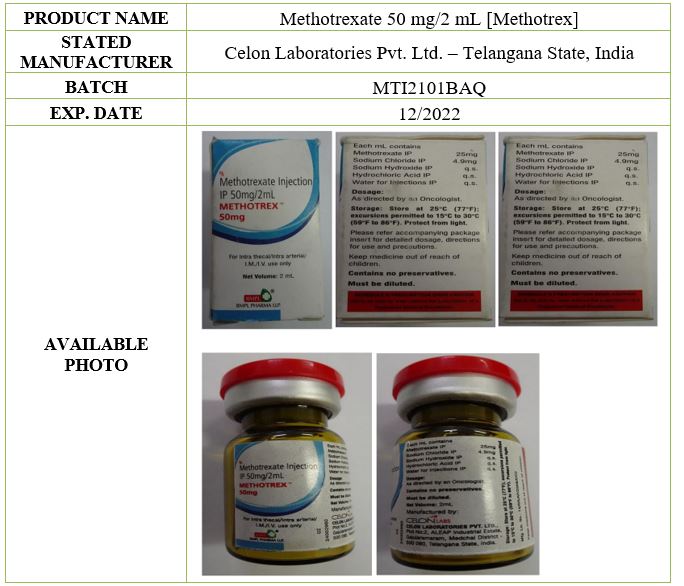
The FDA strongly advises the public to be vigilant on the circulation of this substandard drug product which was found positive with Pseudomonas aeruginosa. A substandard drug product are products that fail to meet either their quality standards or specifications. The stated manufacturer has confirmed that the batch number, manufacturing and expiry dates referenced above corresponds to their internal records. To date, the manufacturer have no access to the samples of the suspect products for their own confirmatory testing.
Methotrexate is a chemotherapy agent and immune system suppressant. It may be given intrathecal, intramuscular, intravenous, or intra-arterial routes. Patients receiving methotrexate treatment may have weakened immune systems and be more vulnerable to opportunistic infections.
Pseudomonas aeruginosa bloodstream infection is a serious infection that may lead to death and any product that has any contamination and is administered directly in the body would present serious risks to patients.
This is to emphasize that Methotrexate 50 mg/2mL Solution for Injection [Methotrex] is not registered with FDA. However, it is important to detect and remove this product from circulation to prevent harm to patients.
Therefore, all Local Government Units (LGU) and Law Enforcement Agencies (LEAs), after the issuance of this advisory, are requested to ensure that this substandard drug product is not sold or not administered to patients in their localities or areas of jurisdiction.
For more information and inquiries, please e-mail us at [email protected]. To report unauthorized sale, or distribution of the abovementioned, kindly e-mail us via [email protected]. You may also call the Center for Drug Regulation and Research at telephone number (02) 8809-5596.
Dissemination of the information to all concerned is highly requested.



