All healthcare professionals and the general public are hereby advised by the Food and Drug Administration (FDA) regarding the voluntary recall by the marketing authorization holder (MAH) on the affected batches of the subject product from the market. The details of the products are as follows:
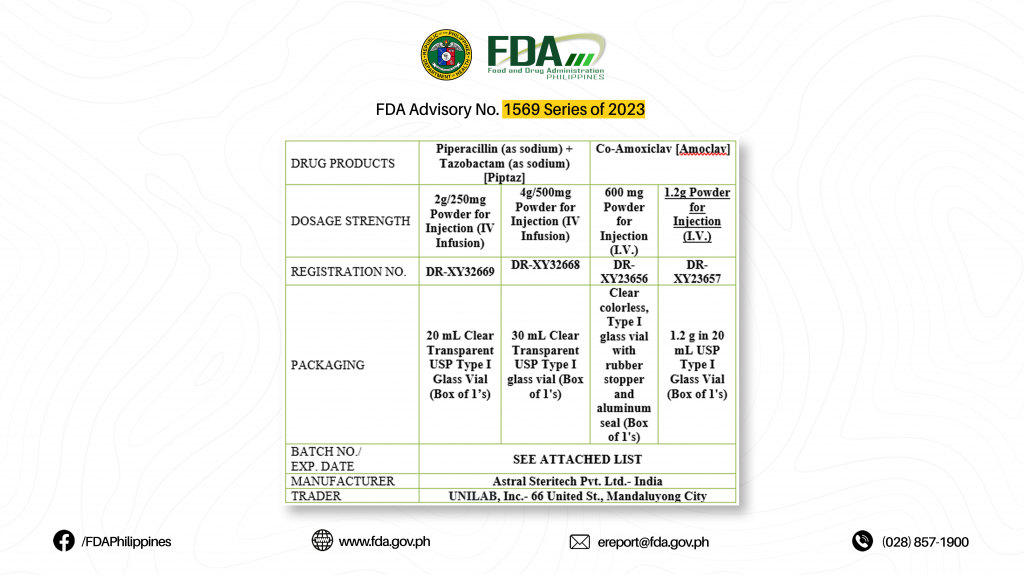
| DRUG PRODUCTS | Piperacillin (as sodium) + Tazobactam (as sodium) [Piptaz] | Co-Amoxiclav [Amoclav] | ||
| DOSAGE STRENGTH | 2g/250mg Powder for Injection (IV Infusion) | 4g/500mg Powder for Injection (IV Infusion) | 600 mg Powder for Injection (I.V.) | 1.2g Powder for Injection (I.V.) |
| REGISTRATION NO. | DR-XY32669 | DR-XY32668 | DR-XY23656 | DR-XY23657 |
| PACKAGING | 20 mL Clear Transparent USP Type I Glass Vial (Box of 1’s) | 30 mL Clear Transparent USP Type I glass vial (Box of 1’s) | Clear colorless, Type I glass vial with rubber stopper and aluminum seal (Box of 1’s) | 1.2 g in 20 mL USP Type I Glass Vial (Box of 1’s) |
| BATCH NO./
EXP. DATE |
SEE ATTACHED LIST | |||
| MANUFACTURER | Astral Steritech Pvt. Ltd.- India | |||
| TRADER | UNILAB, Inc.- 66 United St., Mandaluyong City | |||
Piperacillin/Tazobactam combines a bactericidal antibiotic with a beta-lactamase inhibitor. It is used for the treatment of moderate to severe infections caused by piperacillin-resistant, piperacillin/tazobactam-susceptible, and beta-lactamase producing strains of microorganisms. Co-amoxiclav is an antibiotic used for bacterial infections. It contains amoxicillin (an antibiotic from the penicillin group of medicines) mixed with clavulanic acid.
The MAH pursued the voluntary recall of the drug product due to an Out-of-Specification (OOS) data observed during a routine environmental monitoring at the manufacturer’s facility located in Vadodara, Gujarat, India. Due to the Good Manufacturing Practice (GMP) issues on the manufacturer’s sterile facilities, the integrity of the specific batches produced can no longer be assured and could relatively cause health risks to the patients. Thus, the stated batches present quality and safety concerns.
Distributors, hospitals, retailers, pharmacies, or clinics that have the affected lot of the drug product are therefore instructed to discontinue further distribution, sale, and use. All consumers are likewise advised not to purchase or use the affected product batches and may contact UNILAB, Inc through [email protected] for any question or additional information regarding the recall.
All Local Government Units (LGU) and Law Enforcement Agencies (LEAs) are requested to ensure that the affected product batches are not sold or made available in their localities or areas of jurisdiction.
For more information and inquiries, please e-mail us at [email protected]. To report continuous sale or distribution of the abovementioned, kindly e-mail us via [email protected]. You may also call the Center for Drug Regulation and Research at telephone number (02) 8809-5596. Any suspected adverse reaction experienced from the use of the products should be reported immediately to the FDA through this link: https://primaryreporting.who-umc.org/Reporting/Reporter?OrganizationID=PH and fill-out all of the required fields.
Dissemination of the information to all concerned is requested.