FDA Advisory No. 2020-181-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Hindi Rehistradong Gamot na Green Nature Super Rub with Bee Propolis 20gms

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng hindi rehistradong gamot na: Green Nature Super Rub with Bee Propolis 20gms Napatunayan sa pamamagitan […]
FDA Advisory No. 2020-181 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product Green Nature Super Rub with Bee Propolis 20gms

The Food and Drug Administration (FDA) advises the public against the purchase and use of the unregistered drug product: Green Nature Super Rub with Bee Propolis 20gms FDA Post-Marketing Surveillance (PMS) […]
FDA Advisory No. 2020-180-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Hindi Rehistradong Gamot na:
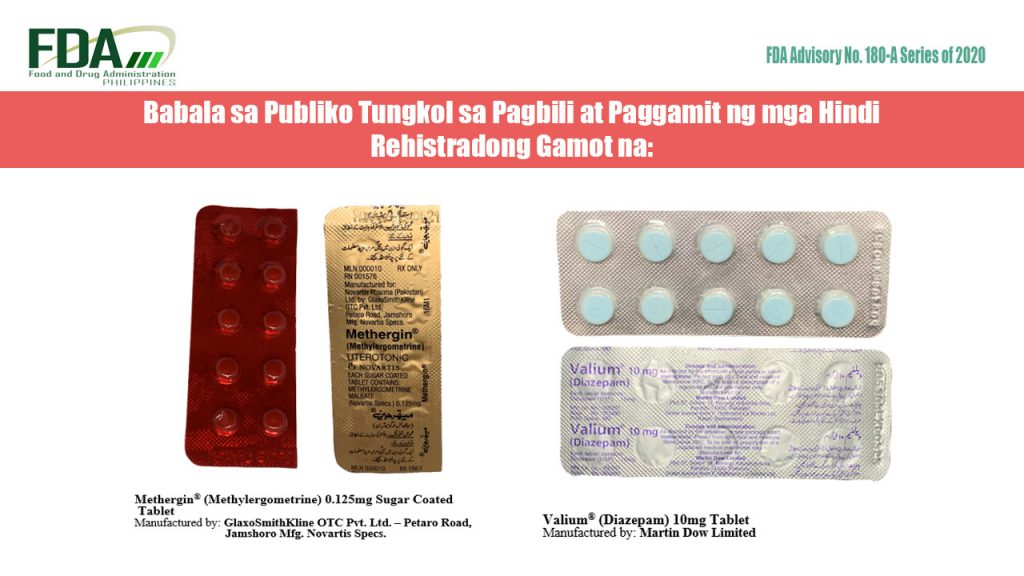
Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga hindi rehistradong gamot na: Methergin® (Methylergometrine) 0.125mg Sugar Coated Tablet Valium® (Diazepam) 10mg Tablet Napatunayan sa […]
FDA Advisory No. 2020-180 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:
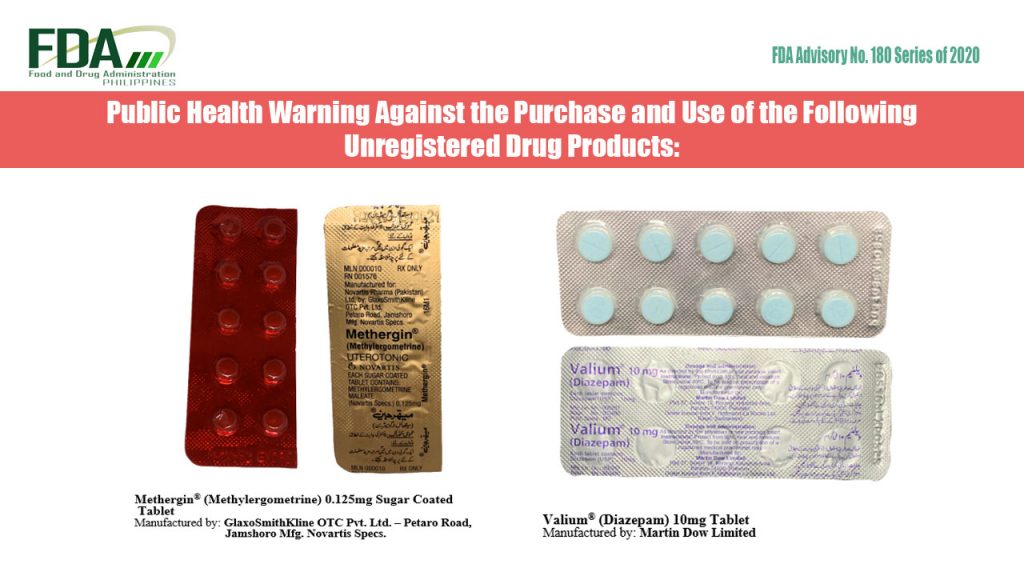
The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered drug products: Methergin® (Methylergometrine) 0.125mg Sugar Coated Tablet Valium® (Diazepam) 10mg Tablet FDA Post-Marketing […]
FDA Circular No. 2020-003 || Guidelines for Pharmaceutical Industry on Pharmacovigilance

to continue reading, click the attachment below… Attachment:-> FDA Circular No.2020-003
The Center for Device Regulation, Radiation Health, and Research shall start receiving the applications based on the ASEAN Harmonized Technical Requirements on 13 March 2020.

FDA Advisory No. 2020-179-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Hindi Rehistradong Gamot na:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng hindi rehistadong mga gamot na: Agua Oxigenada F.E.U. XIV-10 Vols 120mL Agua Oxigenada F.E.U. XIV-10 […]
FDA Advisory No. 2020-179 || Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products:

The Food and Drug Administration (FDA) advised the public against the purchase and use of the following unregistered drug products: Agua Oxigenada F.E.U. XIV-10 Vols 120mL Agua Oxigenada F.E.U. XIV-10 […]
FDA Advisory No. 2020-175 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: AMAZING! KANGEN WATER FLOR’S PASALUBONG TREAT ATO KINI BARQUIRON DE CASHEW FLOR’S […]
FDA Advisory No. 2020-172 || Public Health Warning Against the Purchase and Consumption of the following Unregistered Food Products:

The Food and Drug Administration (FDA) warns the public from purchasing and consuming the following unregistered food products: HARVEY BANANA ROSS SOFT CANDY KITCHEN PANTRY BERYL’S MILK CHOCO JERSEYS’S PEANUT […]



