FDA Advisory No.2024-0661 || Public Health Warning Against the Purchase and Use of the Following Unnotified EIGHTMED Medical Device Products:

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device products: 1. “COMMODE WHEELCHAIR BLACK” 2. “COMMODE […]
FDA Advisory No.2024-0660 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “MEDICAL DEPOT URINE BAG”
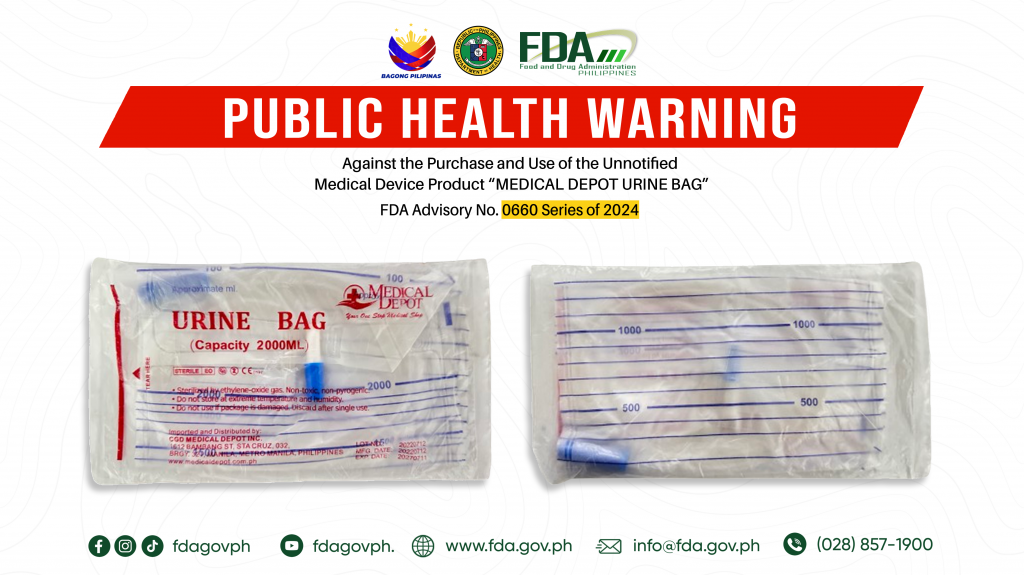
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. MEDICAL DEPOT URINE BAG The […]
FDA Advisory No.2024-0659 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Product “SURGITECH HYPO-ALLERGENIC SURGICAL GLOVES POWDER FREE NATURAL RUBBER LATEX”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product: 1. SURGITECH HYPO-ALLERGENIC SURGICAL GLOVES POWDER […]
FDA Advisory No.2024-0658 || Public Health Warning Against the Purchase and Use of the Unregistered Medical Device Product “SURESHOT DISPOSABLE SYRINGE” with Expired Certificate of Product Registration (CPR)
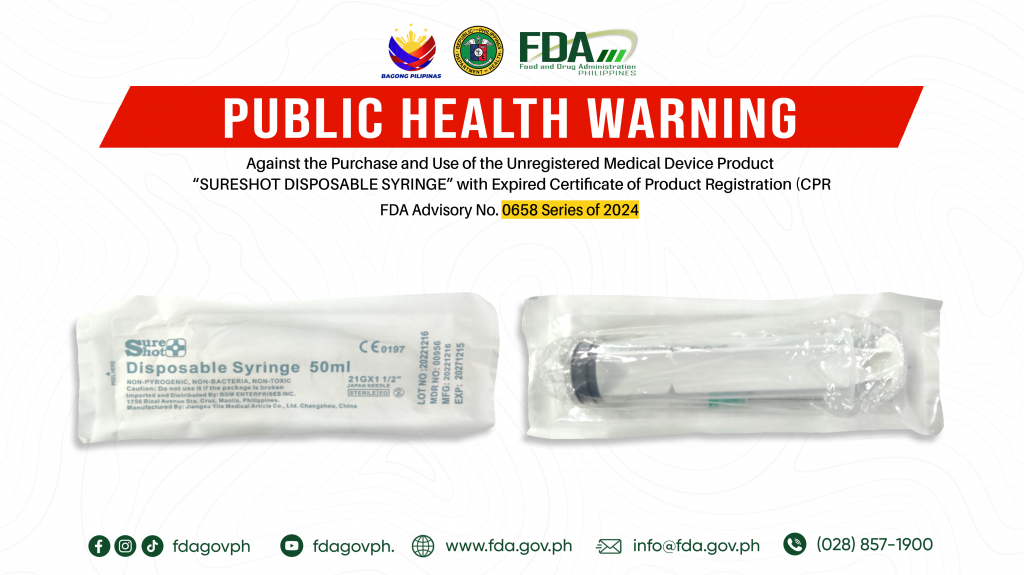
The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unregistered medical device product with Expired Certificate of Product Registration […]
FDA Advisory No.2024-0657 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “YOSHI ADULT DIAPERS”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. YOSHI ADULT DIAPERS The FDA […]
FDA Advisory No.2024-0655 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “KOFUKU HEALTH MASSAGER TENS THERAPY”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. KOFUKU HEALTH MASSAGER TENS THERAPY […]
FDA Advisory No.2024-0652 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “VANTAGE POWDER-FREE NITRILE EXAMINATION GLOVES”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. VANTAGE POWDER-FREE NITRILE EXAMINATION GLOVES […]
FDA Advisory No.2024-0619 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “SAFEWEAR DISPOSABLE EXAMINATION GLOVES”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. SAFEWEAR DISPOSABLE EXAMINATION GLOVES The […]
FDA Advisory No.2024-0570 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “RX DR. CARE MEDICAL COMPRESSION STOCKINGS”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. RX DR. CARE MEDICAL COMPRESSION […]
FDA Advisory No.2024-0569 || Public Health Warning Against the Purchase and Use of the Unnotified Medical Device Product “PRIME ABDOMINAL BINDER”

The Food and Drug Administration (FDA) warns all healthcare professionals and the general public NOT TO PURCHASE AND USE the unnotified medical device product: 1. PRIME ABDOMINAL BINDER The FDA […]



