Announcement || CDRRHR – LRD Workshop and System Update on the Regulation of Medical Device Establishments and Products on 13-15 March 2024

Please be informed that the Center for Device Regulation, Radiation Health, and Research – Licensing and Registration Division (CDRRHR-LRD) will be having its Workshop and System Updates on the Regulation […]
Announcement || CDRRHR 2023 YEAR-END PERFORMANCE REVIEW AND STRATEGIC PLANNING FOR CY 2024

Please be informed that the Center for Device Regulation, Radiation Health, and Research (CDRRHR) will be conducting its Year-End Performance Review and Strategic Planning for CY 2024 on 04 – […]
Announcement || NOTICE OF VIRTUAL PUBLIC HEARING

As part of the mandate of the Food and Drug Administration (FDA) to protect public health and safety through regulation of health products including in vitro diagnostic medical devices (IVDs) […]
Announcement || License to Operate Application through the FDA eServices Portal System for Health-Related Devices

The Food and Drug Administration hereby announces that the application for license to operate for health-related devices through the eServices Portal System will resume on 26 July 2021. For establishments […]
Announcement || Notice of Virtual Public Hearing for the Proposed FDA Circular Entitled “Banning of all Mercury-Containing Thermometers, Sphygmomanometers, Liquid Mercury and Dental Amalgam Capsules

In line with the implementation of Administrative Order (AO) No. 2008-0021 entitled “Gradual Phase-out of Mercury in all Philippine Health Care Facilities and Institutions” and AO No. 2020-0020 entitled “Guidelines […]
FDA has released a total of 410 COVID – 19 Test Kits (133 – PCR based, 115 – Rapid Antibody, 73 – Immunoassay and 89 – Others
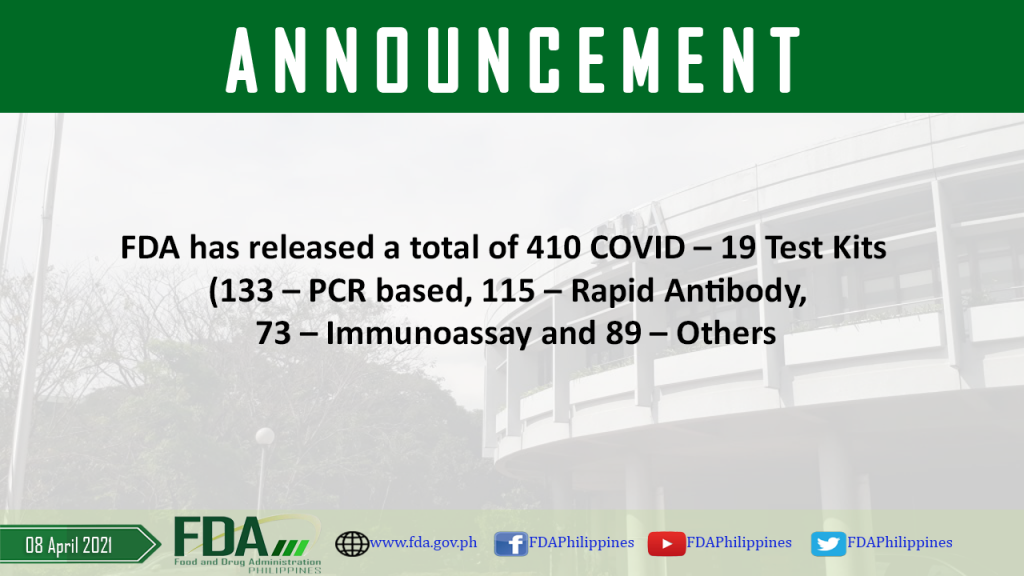
As of 08 April 2021, FDA has released a total of 410 COVID – 19 Test Kits (133 – PCR based, 115 – Rapid Antibody, 73 – Immunoassay and 89– […]
FDA has released a total of 408 COVID – 19 Test Kits (133 – PCR based, 115 – Rapid Antibody, 73 – Immunoassay and 87 – Others
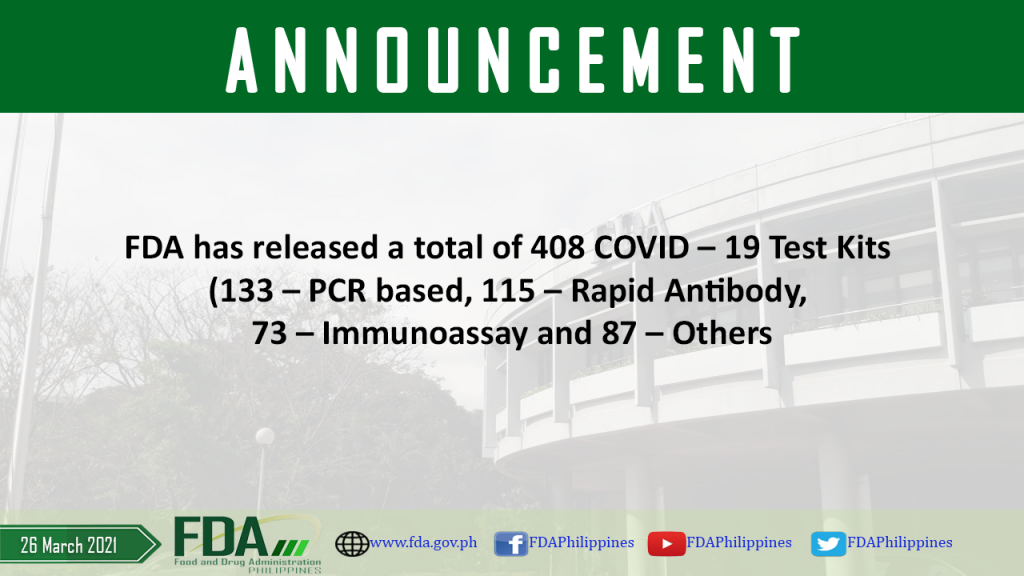
As of 26 March 2021, FDA has released a total of 408 COVID – 19 Test Kits (133 – PCR based, 115 – Rapid Antibody, 73 – Immunoassay and 87 […]
FDA has released a total of 380 COVID – 19 Test Kits (129 – PCR based, 108 – Rapid Antibody, 68 – Immunoassay and 75 – Others).
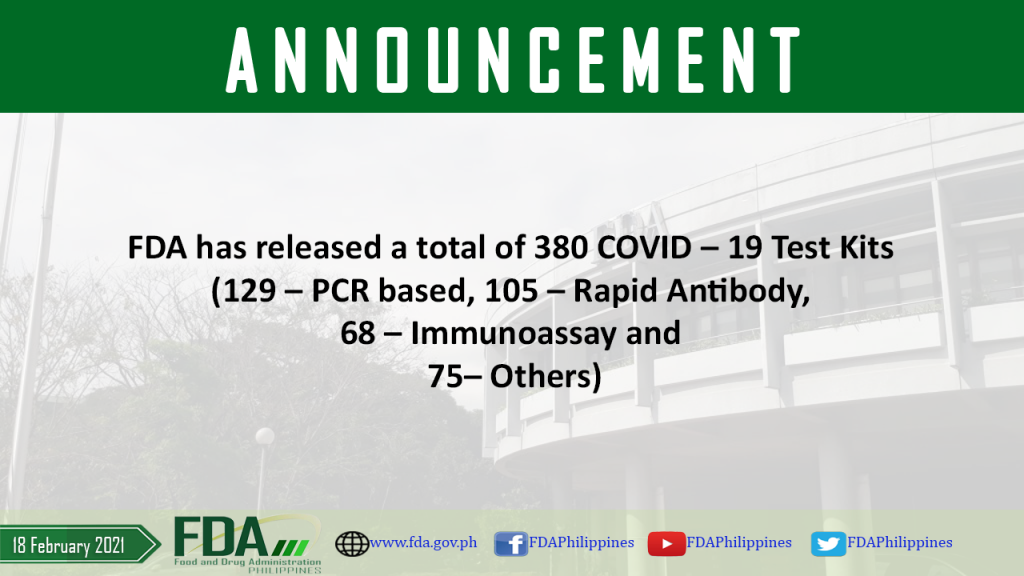
As of 18 February 2021, FDA has released a total of 380 COVID – 19 Test Kits (129 – PCR based, 108 – Rapid Antibody, 68 – Immunoassay and 75 […]
FDA has released a total of 366 COVID – 19 Test Kits (126 – PCR based, 105 – Rapid Antibody, 68 – Immunoassay and 67 – Others).
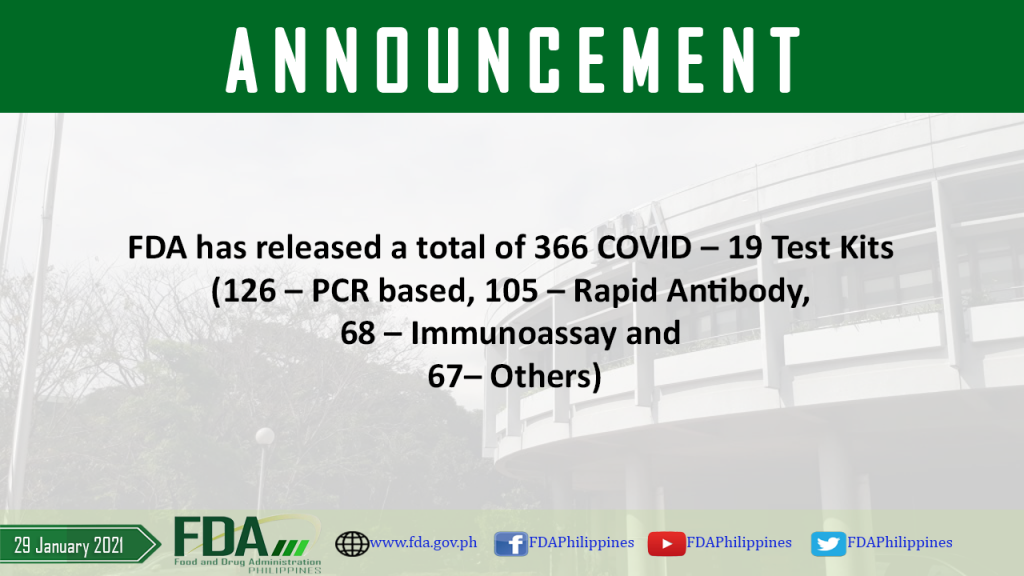
As of 29 January 2021, FDA has released a total of 366 COVID – 19 Test Kits (126 – PCR based, 105 – Rapid Antibody, 68 – Immunoassay and 67 […]
FDA has released a total of 346 COVID – 19 Test Kits (121 – PCR based, 106 – Rapid Antibody, 68 – Immunoassay and 51 – Others).
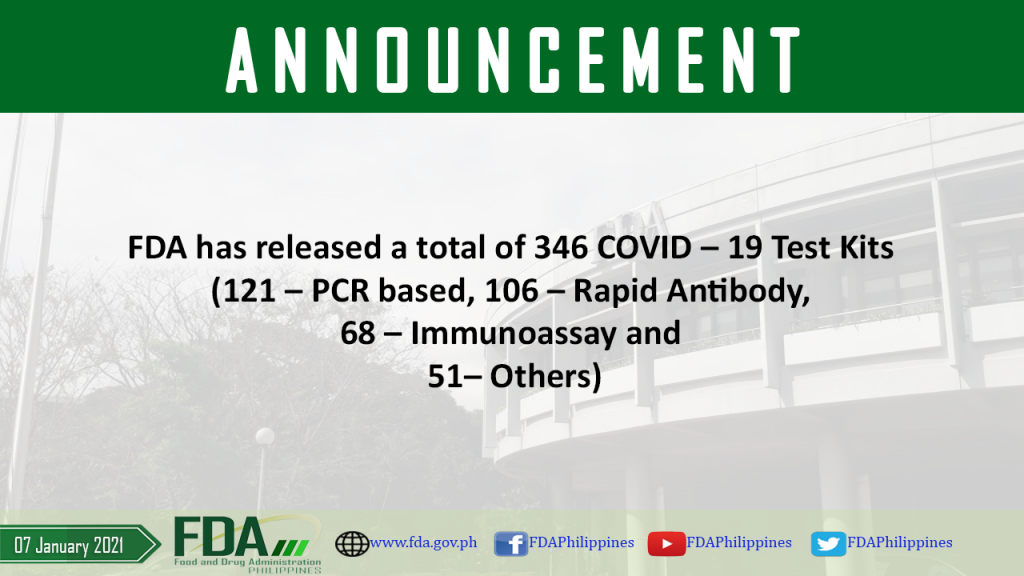
As of 07 January 2021, FDA has released a total of 346 COVID – 19 Test Kits (121 – PCR based, 106 – Rapid Antibody, 68 – Immunoassay and 51 […]



