FDA Advisory No.2023-1713-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Hindi Rehistradong Beterinaryong Gamot na “Himalaya® Since 1930 Immunol Liquid 100 ml”

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng hindi rehistradong beterinaryong gamot na: 1. Himalaya® Since 1930 Immunol Liquid 100 ml Napatunayan sa […]
FDA Advisory No.2023-1713 || Public Health Warning Against the Purchase and Use of the Unregistered Veterinary Drug Product “Himalaya® Since 1930 Immunol Liquid 100 ml”

The Food and Drug Administration (FDA) advises the public against the purchase and use of the unregistered veterinary drug product: 1. Himalaya® Since 1930 Immunol Liquid 100 ml FDA Post-Marketing […]
FDA Advisory No.2023-1590-A || Babala sa Publiko tungkol sa Paggamit ng Beripikadong Pekeng Gamot na “Purified Rabies Vaccine [Vero Cell] (Speeda) 2.5 I.U. and 0.5 mL of solvent Freeze-dried Powder for Injection Intramuscular / Intradermal (I.M./I.D.) Vaccine 5 Vials”
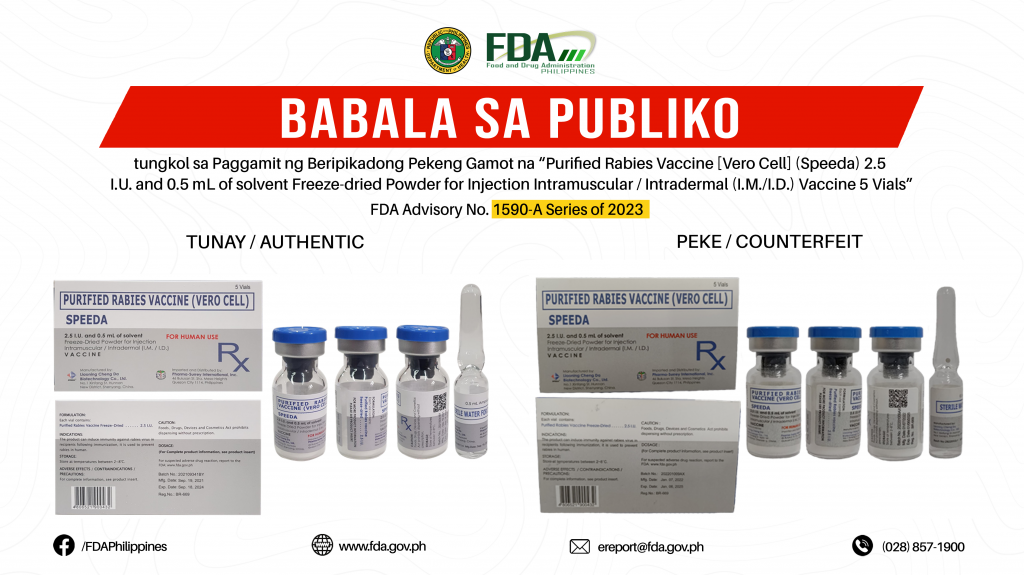
Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng beripikadong pekeng gamot: 1. Purified Rabies Vaccine [Vero Cell] (Speeda) 2.5 I.U. and 0.5 mL […]
FDA Advisory No.2023-1590 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Purified Rabies Vaccine [Vero Cell] (Speeda) 2.5 I.U. and 0.5 mL of solvent Freeze-dried Powder for Injection Intramuscular / Intradermal (I.M./I.D.) Vaccine 5 Vials”
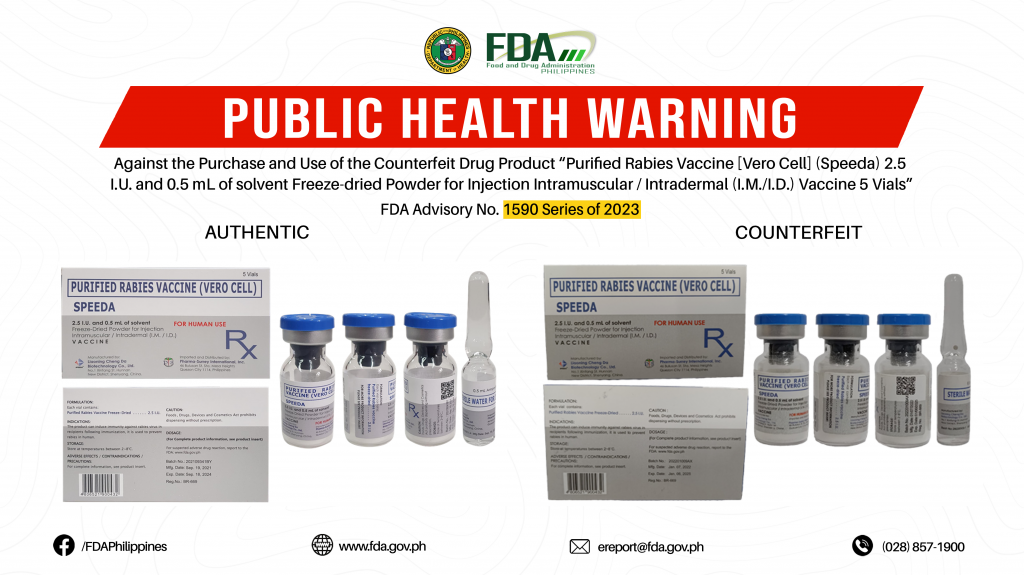
The Food and Drug Administration (FDA) advises the public against the purchase and use of the counterfeit version of the following product: 1. Purified Rabies Vaccine [Vero Cell] (Speeda) 2.5 […]
FDA Advisory No.2023-1589 ||Product Recall of the Specific Batches of Cefepime (as hydrochloride) 1 g Powder for Injection (I.M./I.V.) and Cefepime (as hydrochloride) 2 g Powder for Injection (I.M./I.V.)

All healthcare professionals and the general public are hereby advised by the Food and Drug Administration (FDA) regarding the voluntary recall by the marketing authorization holder (MAH) on the affected […]
FDA Advisory No.2023-1569 || Product Recall of the Specific Batches of Piperacillin (as sodium) + Tazobactam (as sodium) 2g/250mg Powder for Injection (IV Infusion) [Piptaz], Piperacillin (as sodium) + Tazobactam (as sodium) 4g/500mg Powder for Injection (IV Infusion) [Piptaz], Co-Amoxiclav 600 mg Powder for Injection [Amoclav] and Co-Amoxiclav 1.2g Powder for Injection (I.V.) [Amoclav]
All healthcare professionals and the general public are hereby advised by the Food and Drug Administration (FDA) regarding the voluntary recall by the marketing authorization holder (MAH) on the affected […]
FDA Advisory No.2023-1520-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng Hindi Rehistradong Gamot na “Hodaf® Anti-smoke Patch 30’s” Gluta Lipo Brand Protectz Vitamin C + Zinc 500 mg Capsule

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng hindi rehistradong gamot na: 1. Hodaf® Anti-smoke Patch 30’s Napatunayan sa pamamagitan ng isinagawang Post-Marketing […]
FDA Advisory No.2023-1520 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Hodaf® Anti-smoke Patch 30’s”

The Food and Drug Administration (FDA) advises the public against the purchase and use of the unregistered drug product: 1. Hodaf® Anti-smoke Patch 30’s FDA Post-Marketing Surveillance (PMS) activities have […]
FDA Advisory No.2023-1451-A || Babala sa Publiko Tungkol sa Pagbili at Paggamit ng mga Sumusunod na Adulterated at Hindi Rehistradong Gamot:

Pinapayuhan ng Food and Drug Administration (FDA) ang publiko laban sa pagbili at paggamit ng mga sumusunod na adulterated at hindi rehistradong gamot: 1. Alcogenic Isopropyl Alcohol 70% with Moisturizer […]
FDA Advisory No.2023-1451 || Public Health Warning Against the Purchase and Use of the Following Unregistered and Adulterated Drug Products:

The Food and Drug Administration (FDA) advises the public against the purchase and use of the following unregistered and adulterated drug products: 1. Alcogenic Isopropyl Alcohol 70% with Moisturizer 500 […]



