#MedSafetyWeek 2020 Day 1

Umiinom ng gamot ngunit may kakaibang nararamdaman? Baka side effect na yan! I-report sa FDA (https://primaryreporting.who-umc.org/Reporting/Reporter?OrganizationID=PH). Makipagtulugan tungo sa mas ligtas na gamot para sa lahat. #MedSafetyWeek #EveryReportCounts #bePHARMAcoVIGILANTes #PhFDA
AMENDMENT OF THE MECHANICS FOR THE POSTER MAKING COMPETITION FOR 2020 NATIONAL CONSCIOUSNESS WEEK AGAINST COUNTERFEIT MEDICINES (NCWACM)

BACKGROUND The 2020 National Consciousness Week Against Counterfeit Medicines (NCWACM) will be celebrated by the Food and Drug Administration (FDA) on 16 to 20 November 2020 with the theme, “Sa Gitna ng Pandemya, Magtulungan Laban […]
ANNOUNCEMENT || POSTER MAKING COMPETITION FOR 2020 NATIONAL CONSCIOUSNESS WEEK AGAINST COUNTERFEIT MEDICINES (NCWACM)

BACKGROUND By virtue of the Republic Act No. 8203 otherwise known as the “Special Law on Counterfeit Drugs” issued on the 19th of November 1996, this was established to: 1) protect […]
Extension of comments for Draft FDA Circular on Interim Guidelines for the Issuance of Foreign cGMP Clearance

Please be informed that the CDRR is extending the deadline from 10 June 2020 at 3:00PM to 11 June 2020 at 5:00pm of soliciting for comments from the concerned stakeholders on the […]
Development of Drugs and Vaccines
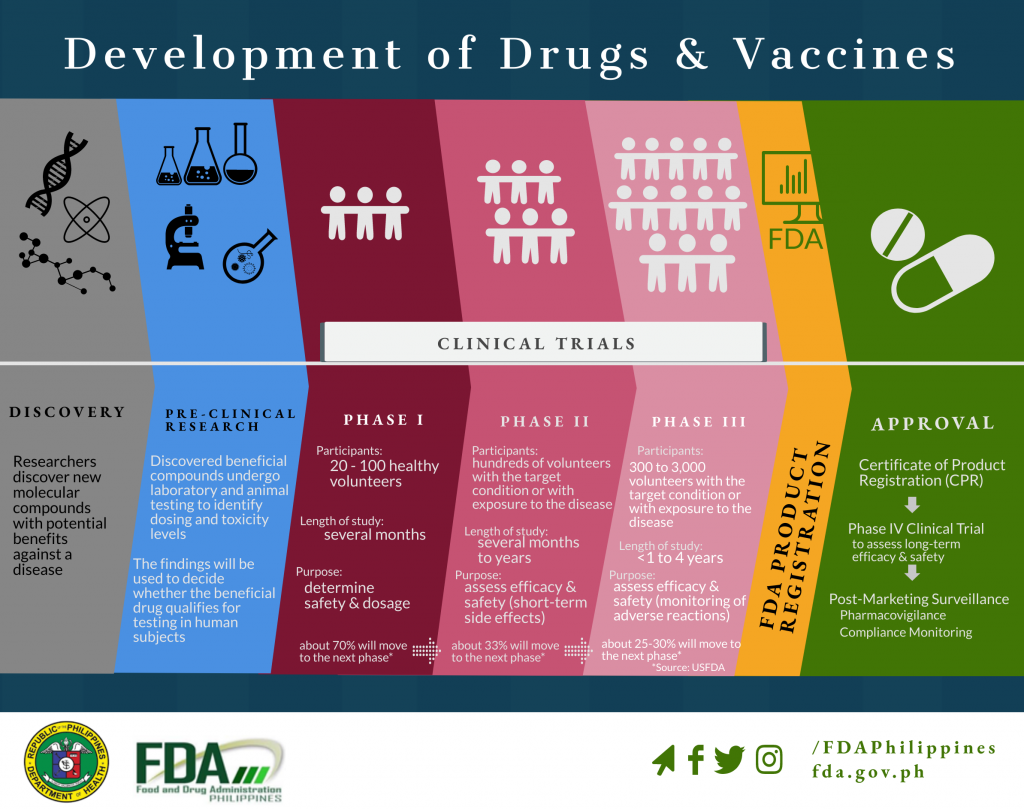
Clinical Trial Application Process

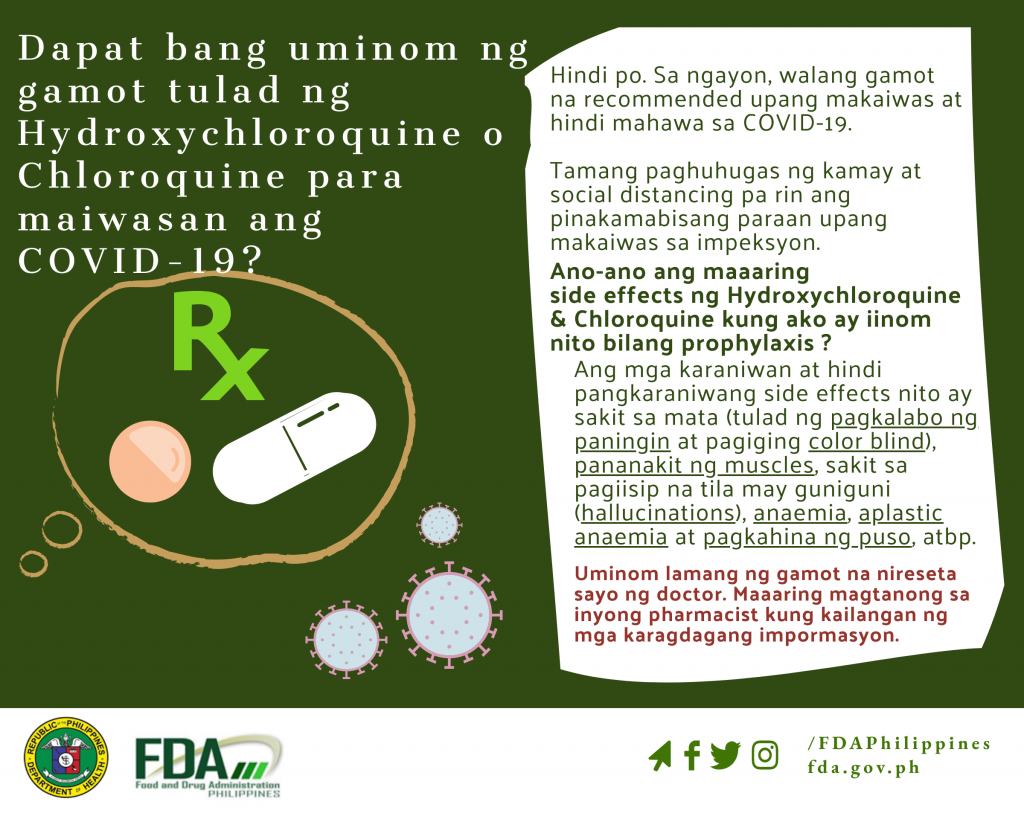
Dapat bang uminom ng gamot tulad ng Hydroxychloroquine o Chloroquine para maiwasan ang COVID-19?
For your Information.
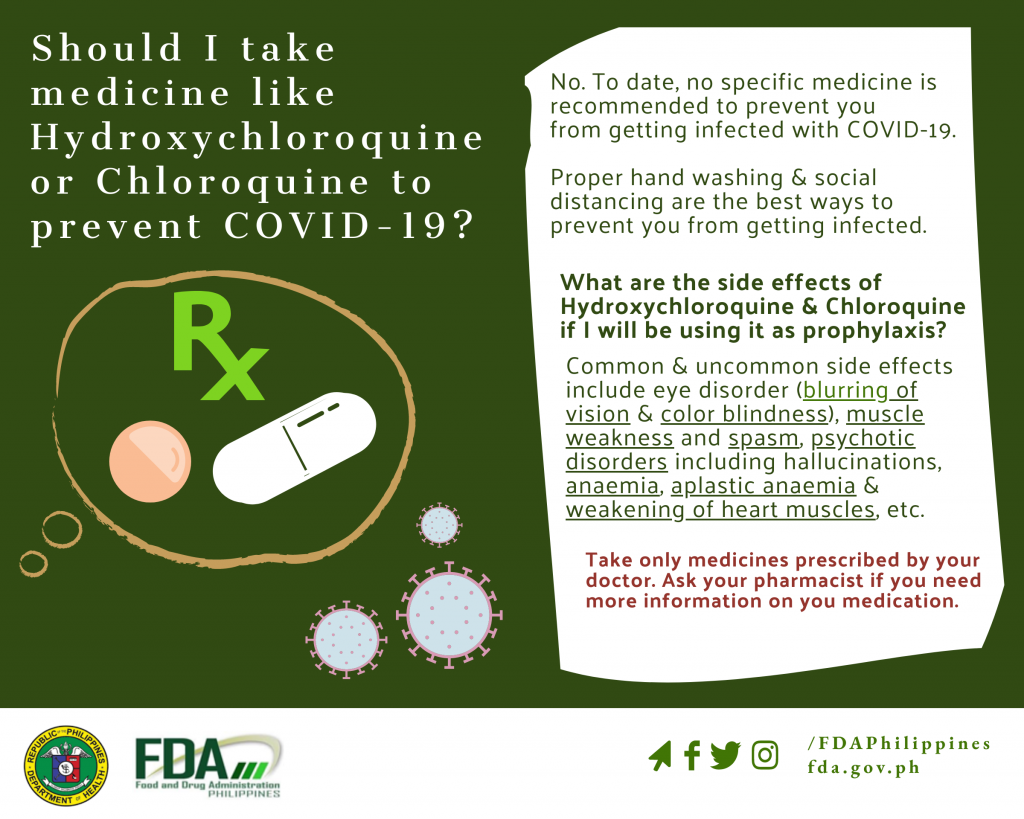
Should I take medicine like Hydroxychloroquine or Chloroquine to prevent COVID-19?
For your Information.
Media Announcement of Seapharma International, Inc. on the Recall of Affected Batches of Enalapril Maleate 10 mg and 20 mg Tablets (Prilsea 10 & Prilsea 20)

SUMMARY Reason for Announcement: Out-of-Specification (Failed Assay) in the Laboratory Analyses Marketing Authorization Holder (MAH): Seapharma International, Inc. Product Description: Enalapril Maleate 10 mg and 20 mg Tablets (Prilsea 10 […]
List of Authorized Biosimilars (as of 1 January 2019)

The first regulation on pharmaceuticals was in 1989 this include registration of vaccines. In 2001 the specific regulation of vaccines and biological products was signed into law, this provides guidance […]



